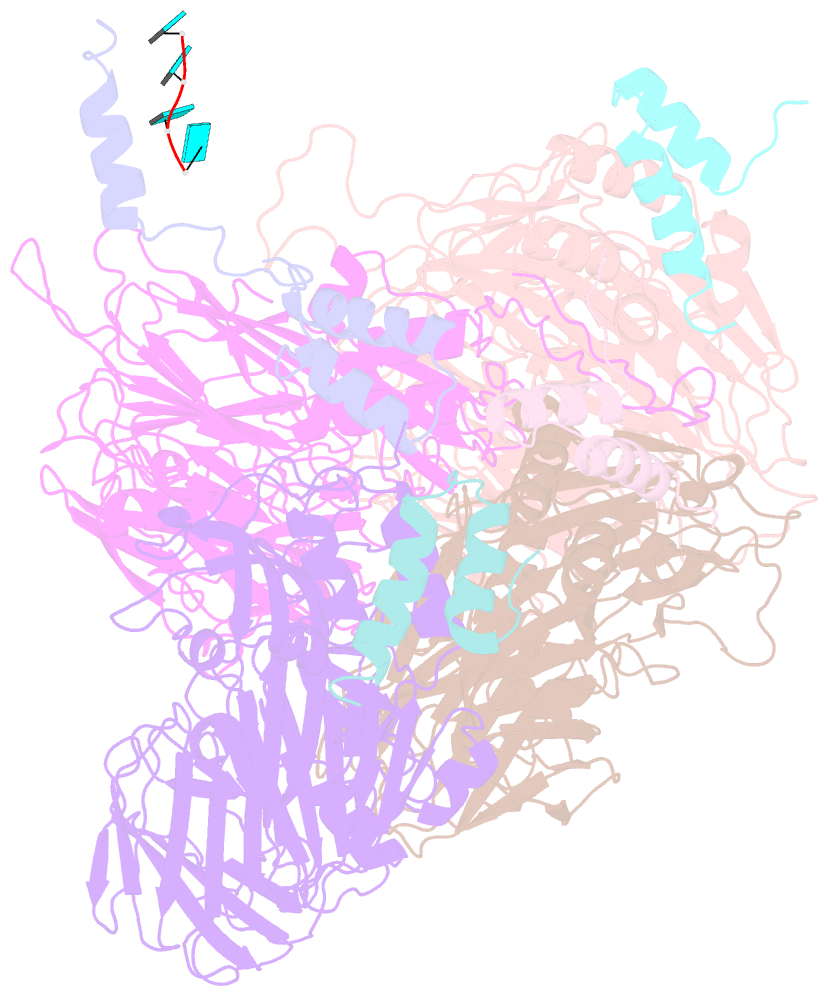
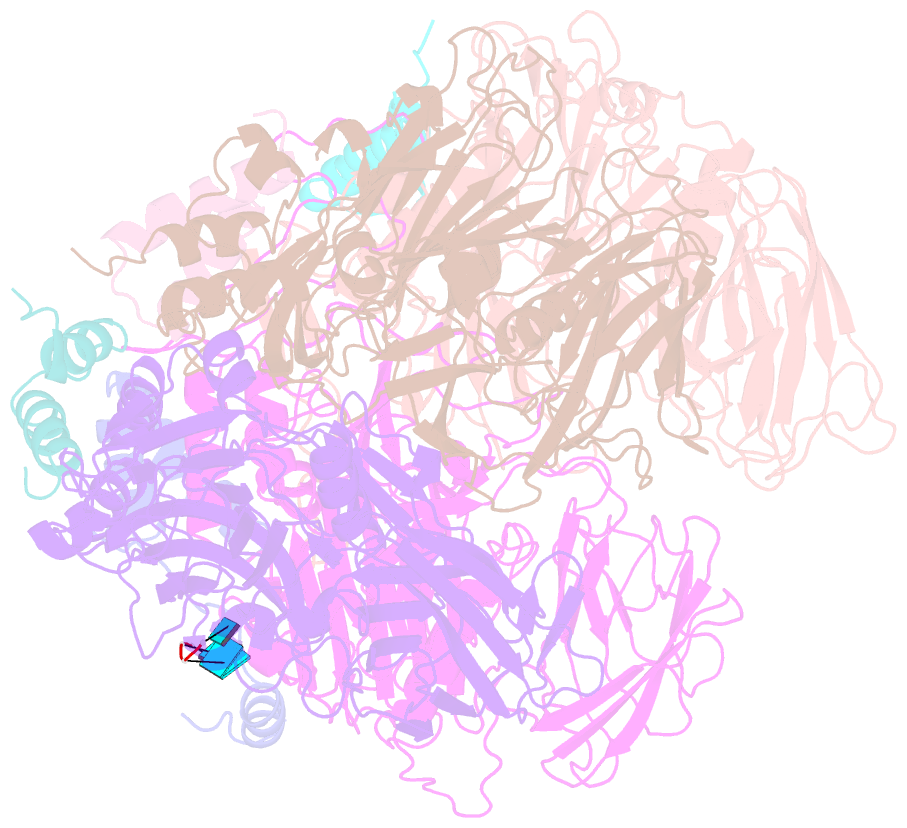
Summary information and primary citation
- PDB-id
- 2qqp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- virus
- Method
- X-ray (3.8 Å)
- Summary
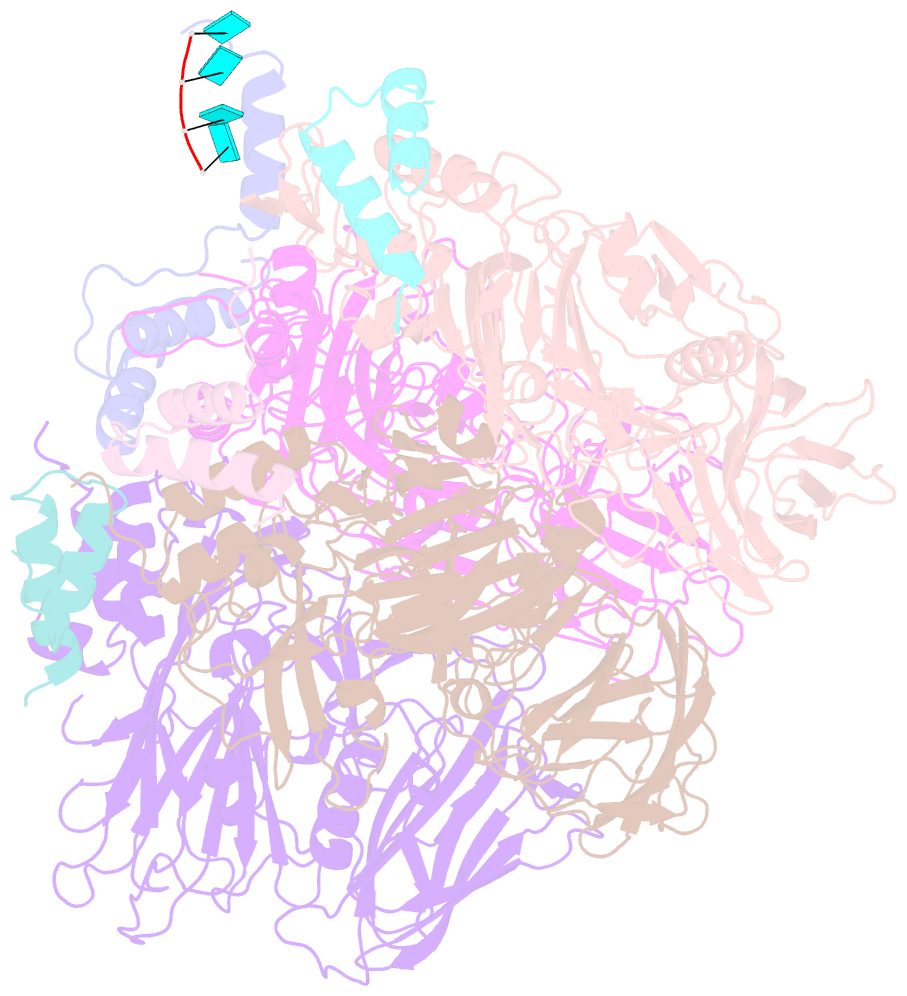
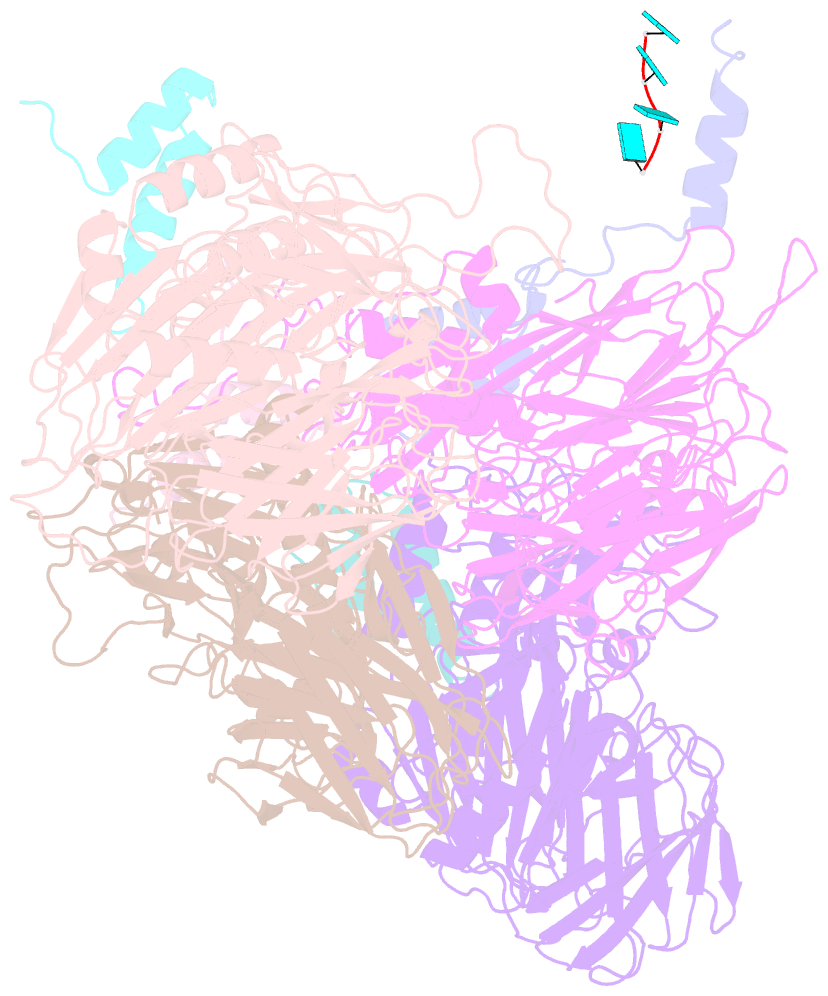
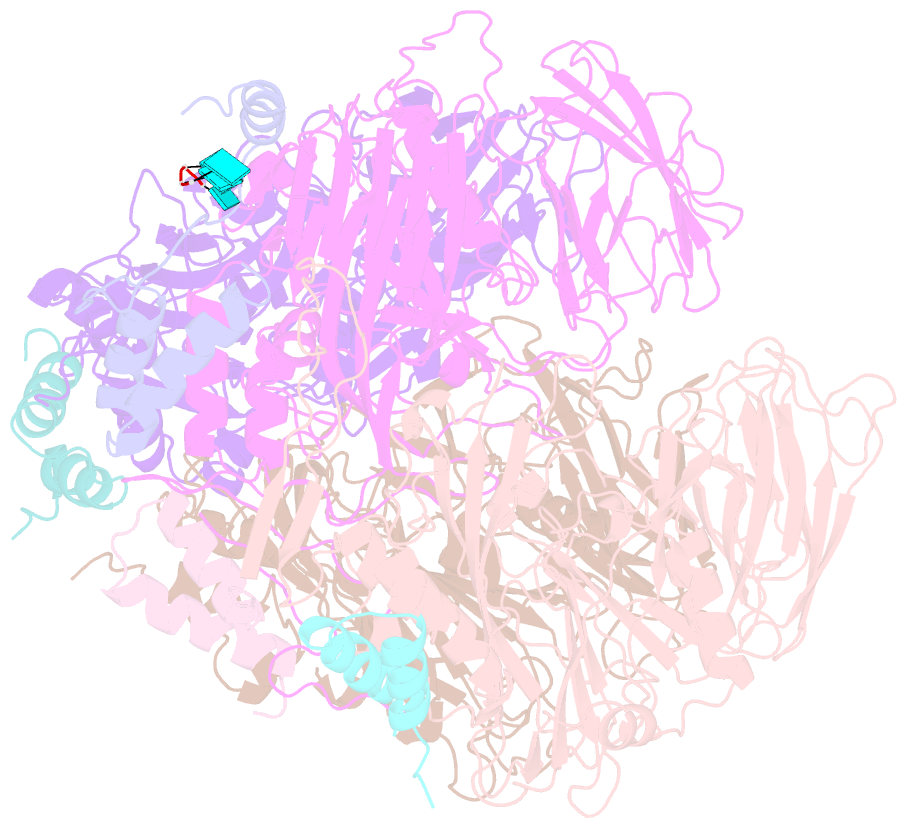
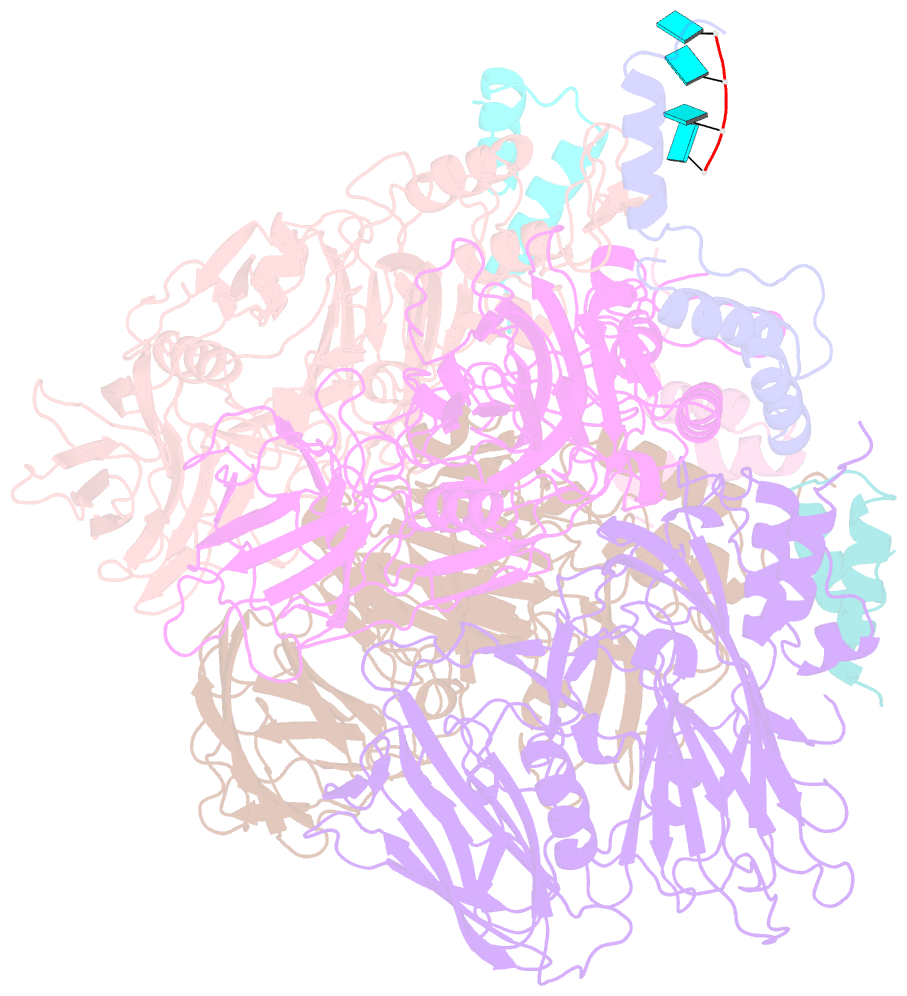
- Crystal structure of authentic providence virus
- Reference
- Speir JA, Taylor DJ, Natarajan P, Pringle FM, Ball LA, Johnson JE (2010): "Evolution in action: N and C termini of subunits in related T = 4 viruses exchange roles as molecular switches." Structure, 18, 700-709. doi: 10.1016/j.str.2010.03.010.
- Abstract
- The T = 4 tetravirus and T = 3 nodavirus capsid proteins undergo closely similar autoproteolysis to produce the N-terminal beta and C-terminal, lipophilic gamma polypeptides. The gamma peptides and the N termini of beta also act as molecular switches that determine their quasi equivalent capsid structures. The crystal structure of Providence virus (PrV), only the second of a tetravirus (the first was NomegaV), reveals conserved folds and cleavage sites, but the protein termini have completely different structures and the opposite functions of those in NomegaV. N termini of beta form the molecular switch in PrV, whereas gamma peptides play this role in NomegaV. PrV gamma peptides instead interact with packaged RNA at the particle two-folds by using a repeating sequence pattern found in only four other RNA- or membrane-binding proteins. The disposition of peptide termini in PrV is closely related to those in nodaviruses, suggesting that PrV may be closer to the primordial T = 4 particle than NomegaV.