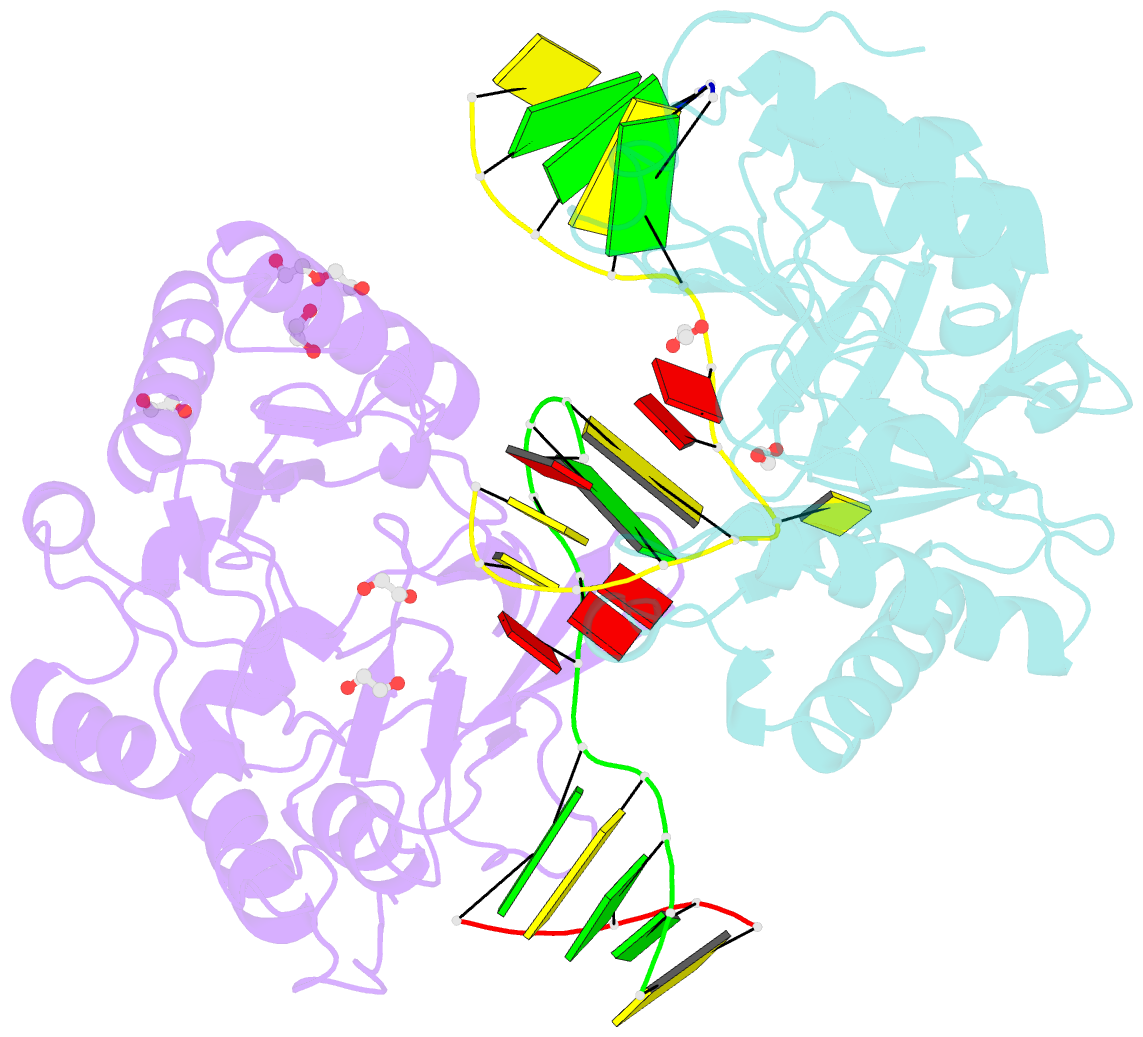
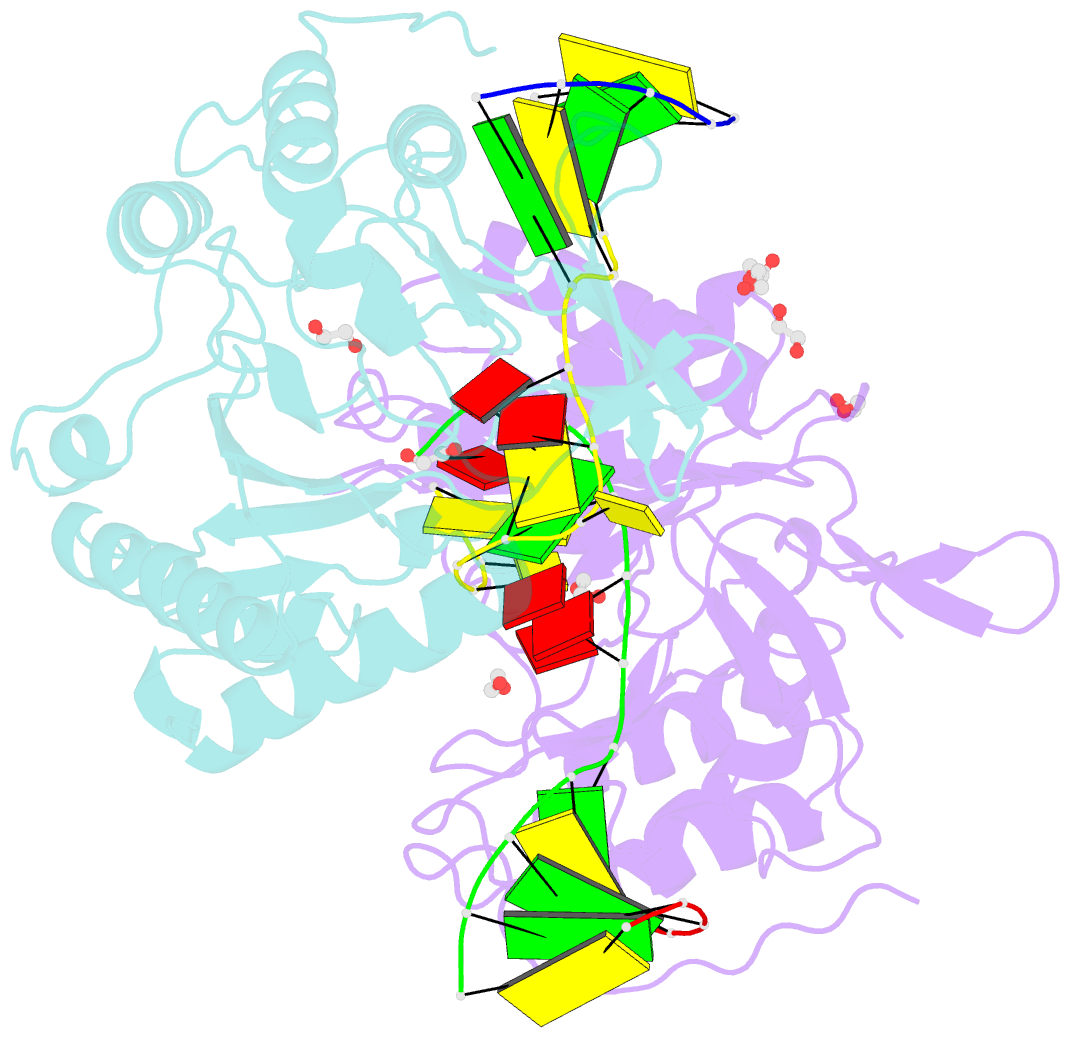
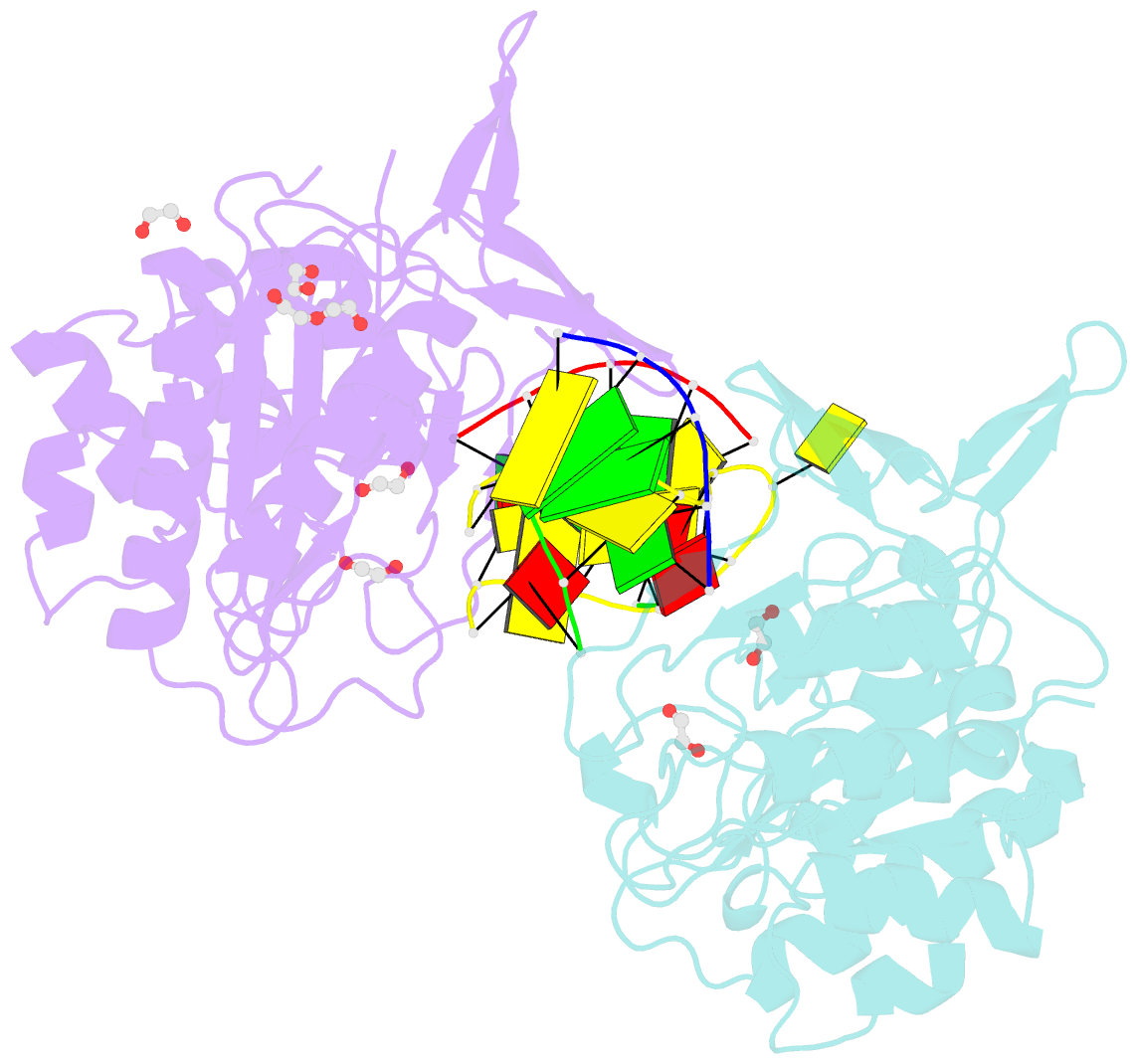
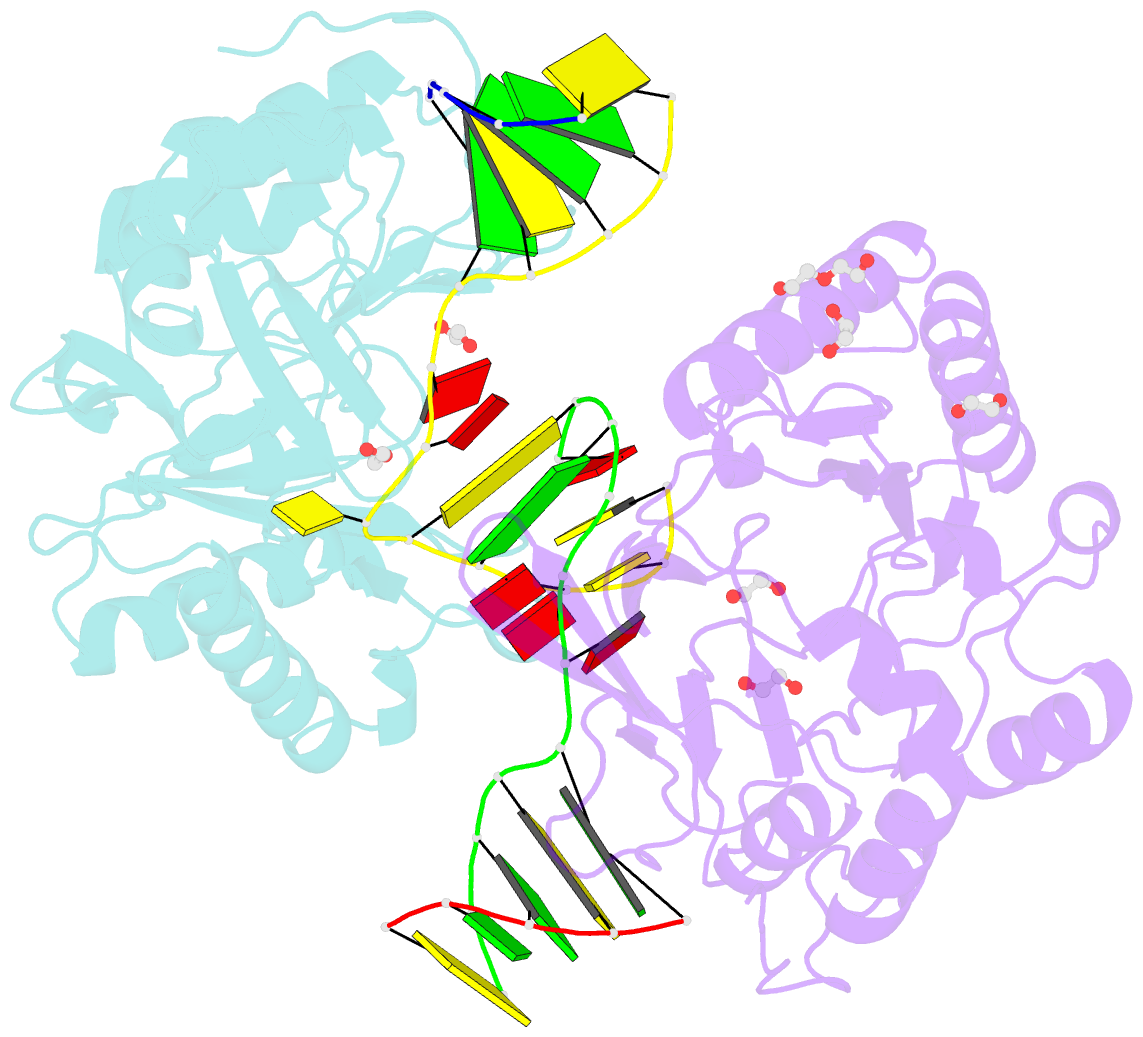
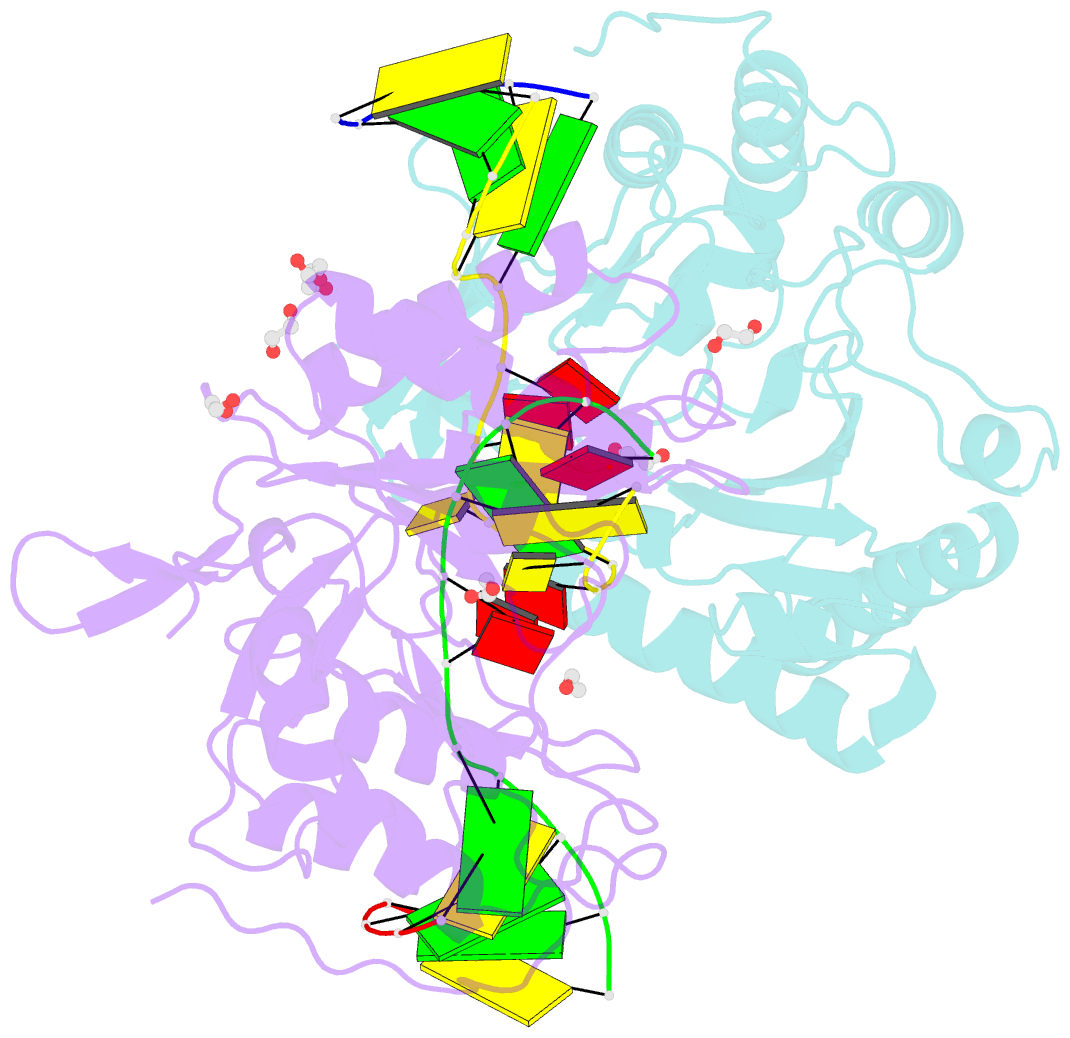
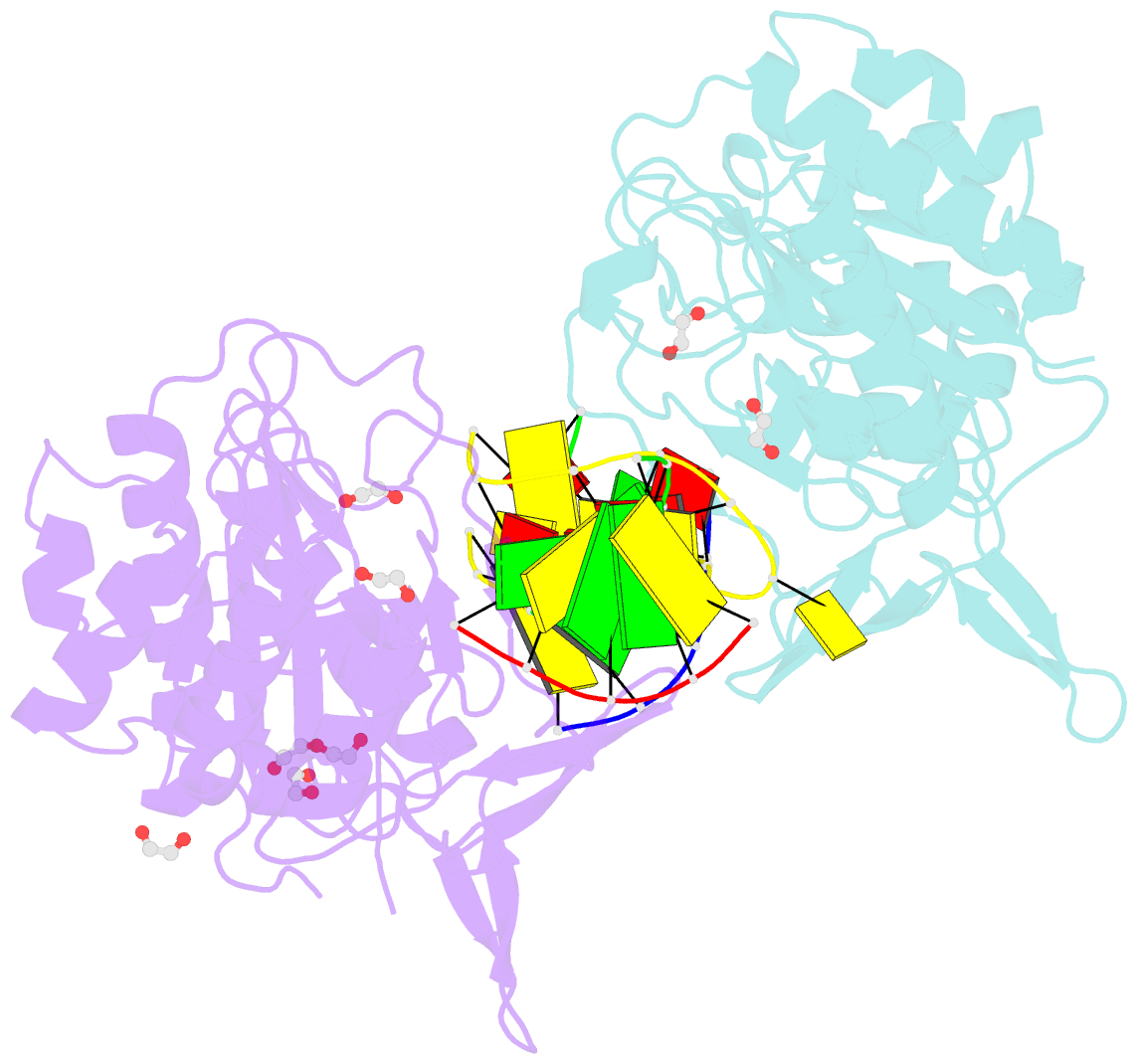
Summary information and primary citation
- PDB-id
- 2r9l; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.4 Å)
- Summary
- Polymerase domain from mycobacterium tuberculosis ligase d in complex with DNA
- Reference
- Brissett NC, Pitcher RS, Juarez R, Picher AJ, Green AJ, Dafforn TR, Fox GC, Blanco L, Doherty AJ (2007): "Structure of a NHEJ polymerase-mediated DNA synaptic complex." Science, 318, 456-459. doi: 10.1126/science.1145112.
- Abstract
- Nonhomologous end joining (NHEJ) is a critical DNA double-strand break (DSB) repair pathway required to maintain genome stability. Many prokaryotes possess a minimalist NHEJ apparatus required to repair DSBs during stationary phase, composed of two conserved core proteins, Ku and ligase D (LigD). The crystal structure of Mycobacterium tuberculosis polymerase domain of LigD mediating the synapsis of two noncomplementary DNA ends revealed a variety of interactions, including microhomology base pairing, mismatched and flipped-out bases, and 3' termini forming hairpin-like ends. Biochemical and biophysical studies confirmed that polymerase-induced end synapsis also occurs in solution. We propose that this DNA synaptic structure reflects an intermediate bridging stage of the NHEJ process, before end processing and ligation, with both the polymerase and the DNA sequence playing pivotal roles in determining the sequential order of synapsis and remodeling before end joining.