Summary information and primary citation
- PDB-id
- 2ru3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- NMR
- Summary
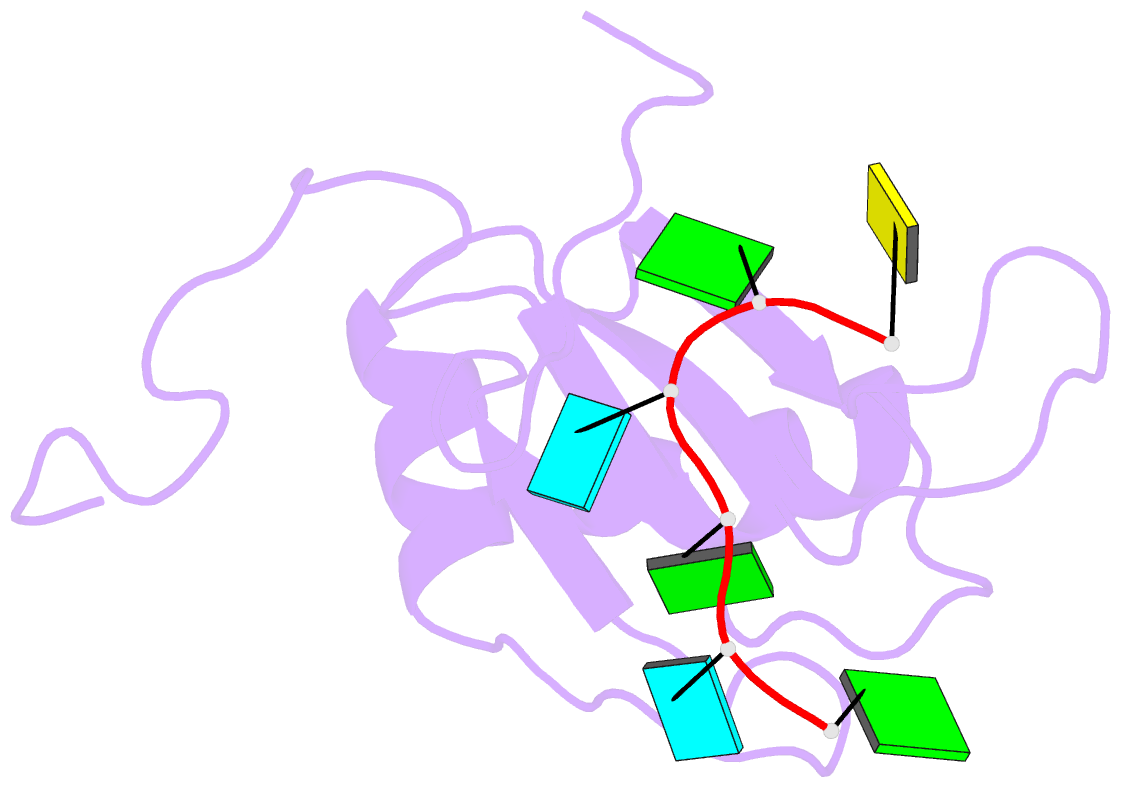
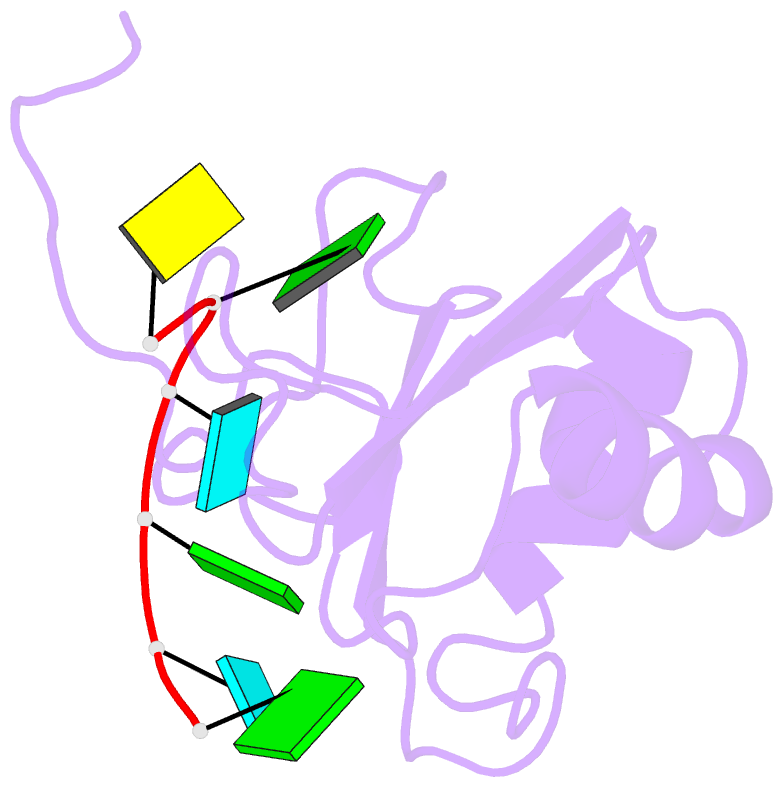
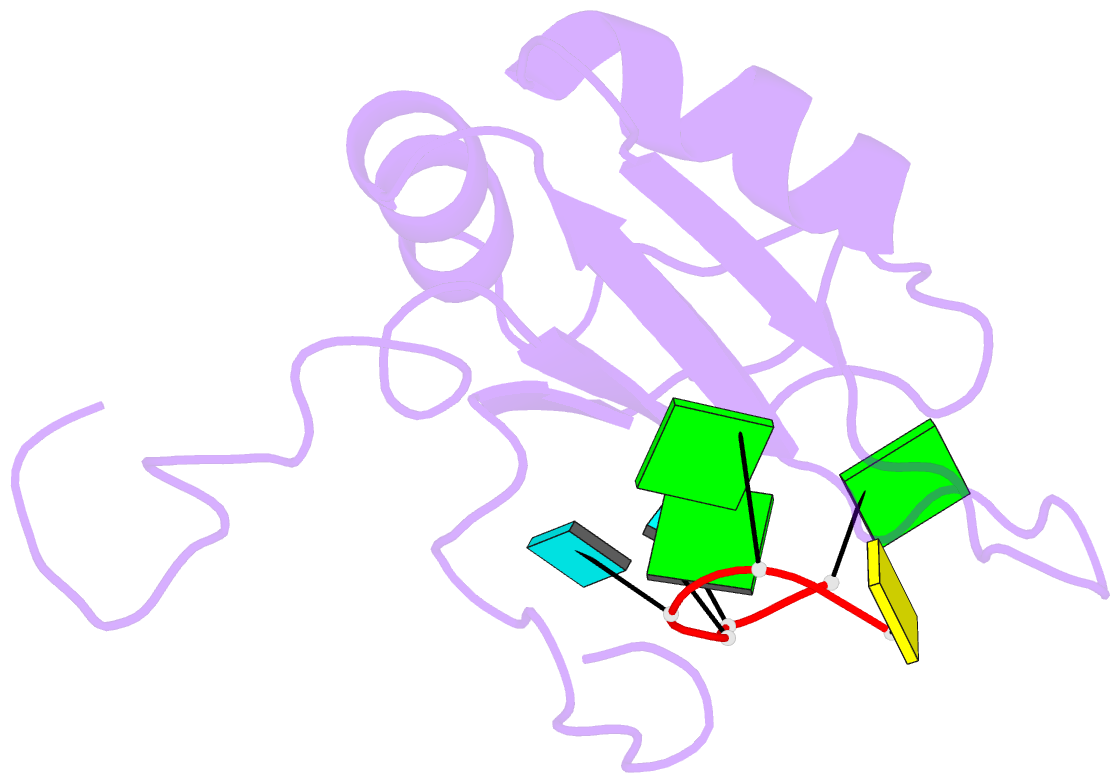
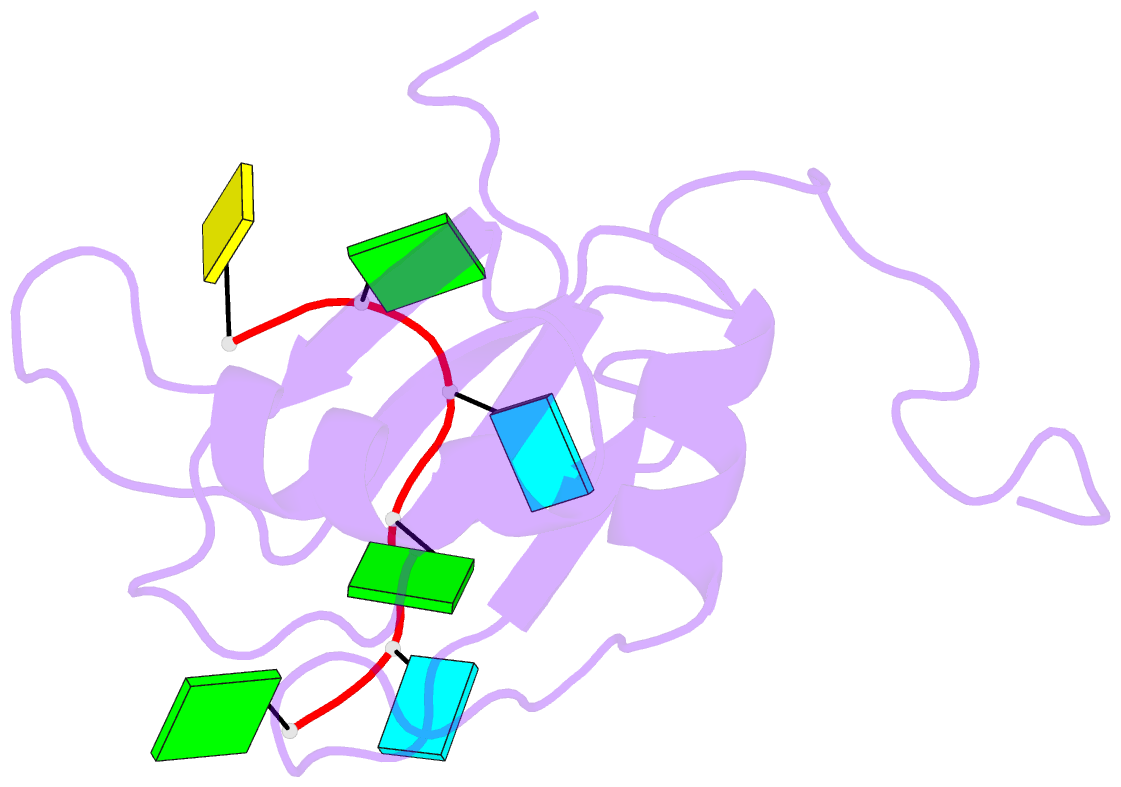
- Solution structure of c.elegans sup-12 rrm in complex with RNA
- Reference
- Kuwasako K, Takahashi M, Unzai S, Tsuda K, Yoshikawa S, He F, Kobayashi N, Guntert P, Shirouzu M, Ito T, Tanaka A, Yokoyama S, Hagiwara M, Kuroyanagi H, Muto Y (2014): "RBFOX and SUP-12 sandwich a G base to cooperatively regulate tissue-specific splicing." Nat.Struct.Mol.Biol., 21, 778-786. doi: 10.1038/nsmb.2870.
- Abstract
- Tissue-specific alternative pre-mRNA splicing is often cooperatively regulated by multiple splicing factors, but the structural basis of cooperative RNA recognition is poorly understood. In Caenorhabditis elegans, ligand binding specificity of fibroblast growth factor receptors (FGFRs) is determined by mutually exclusive alternative splicing of the sole FGFR gene, egl-15. Here we determined the solution structure of a ternary complex of the RNA-recognition motif (RRM) domains from the RBFOX protein ASD-1, SUP-12 and their target RNA from egl-15. The two RRM domains cooperatively interact with the RNA by sandwiching a G base to form the stable complex. Multichromatic fluorescence splicing reporters confirmed the requirement of the G and the juxtaposition of the respective cis elements for effective splicing regulation in vivo. Moreover, we identified a new target for the heterologous complex through an element search, confirming the functional significance of the intermolecular coordination.