Summary information and primary citation
- PDB-id
- 2uwm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- translation
- Method
- X-ray (2.31 Å)
- Summary
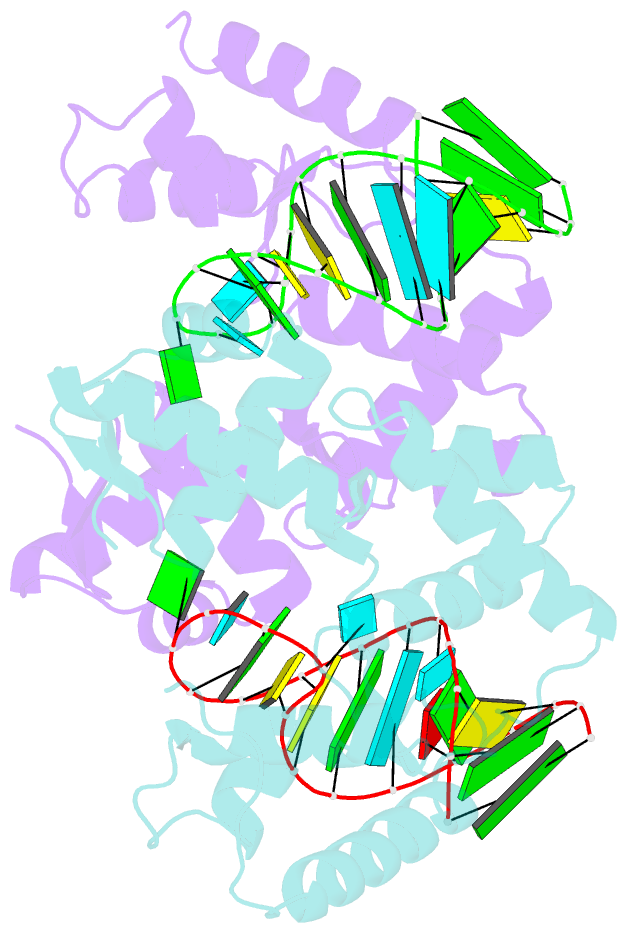
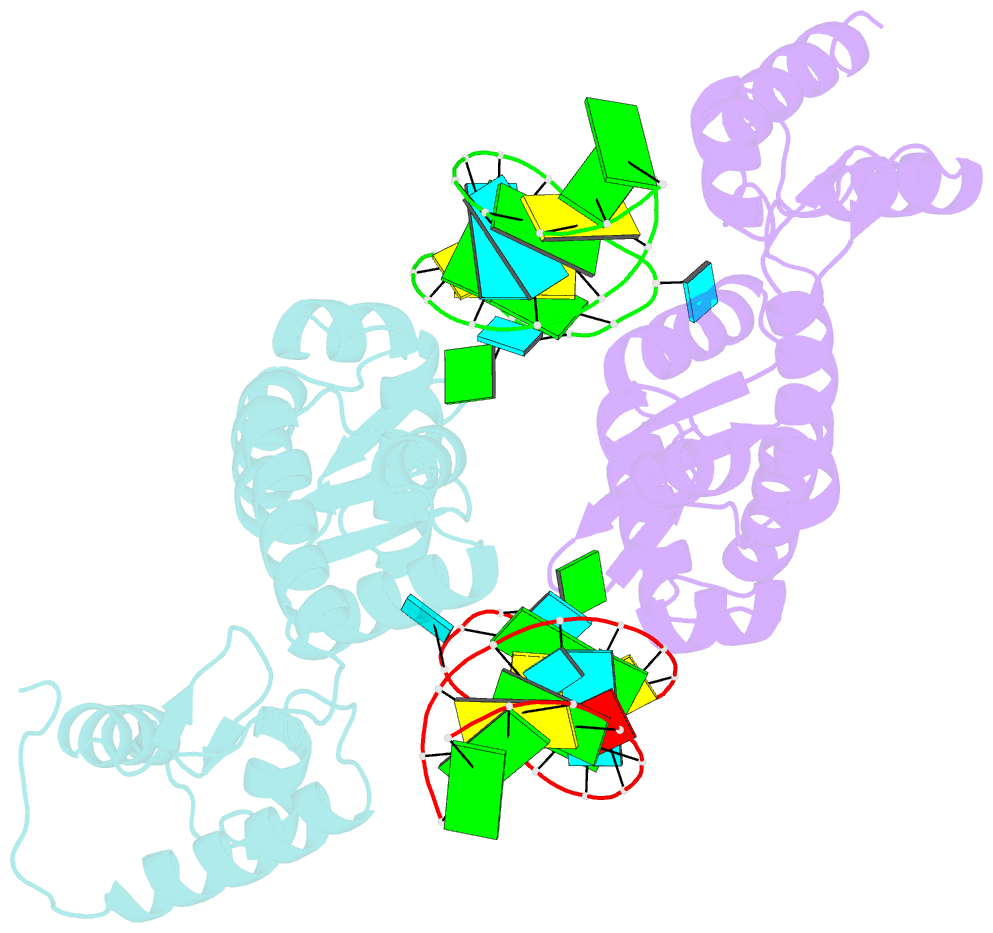
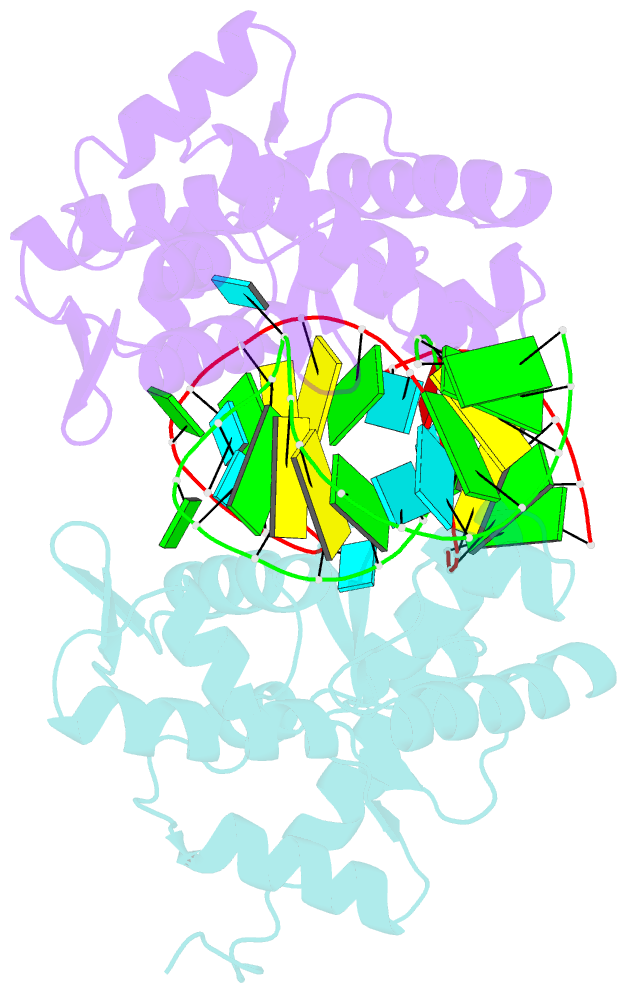
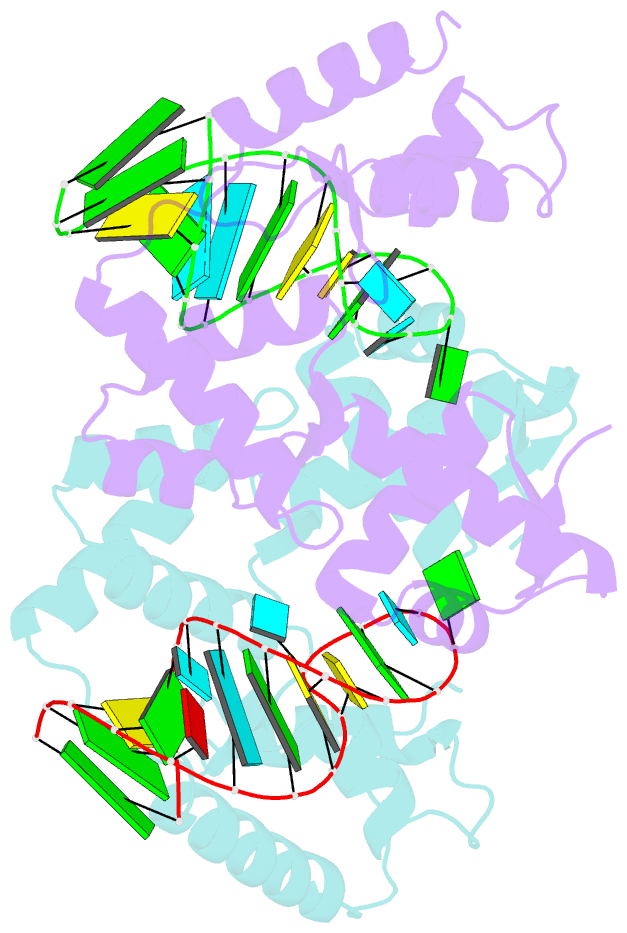
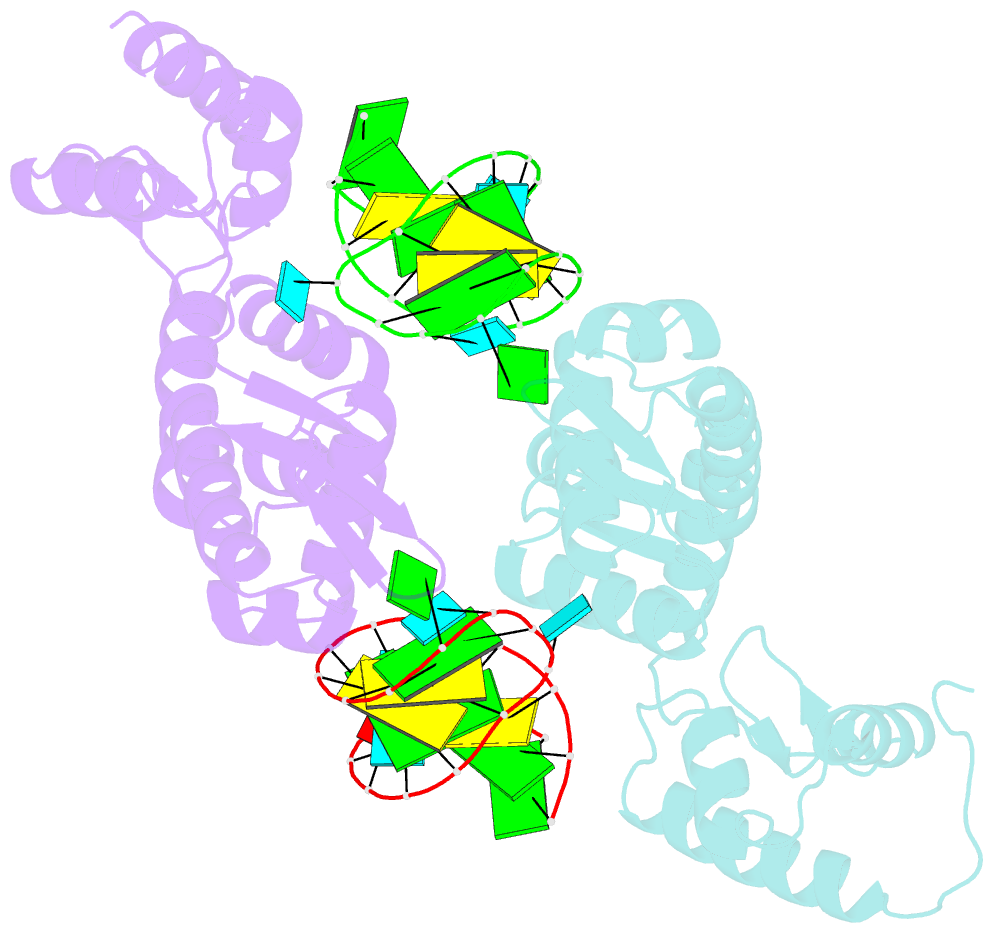
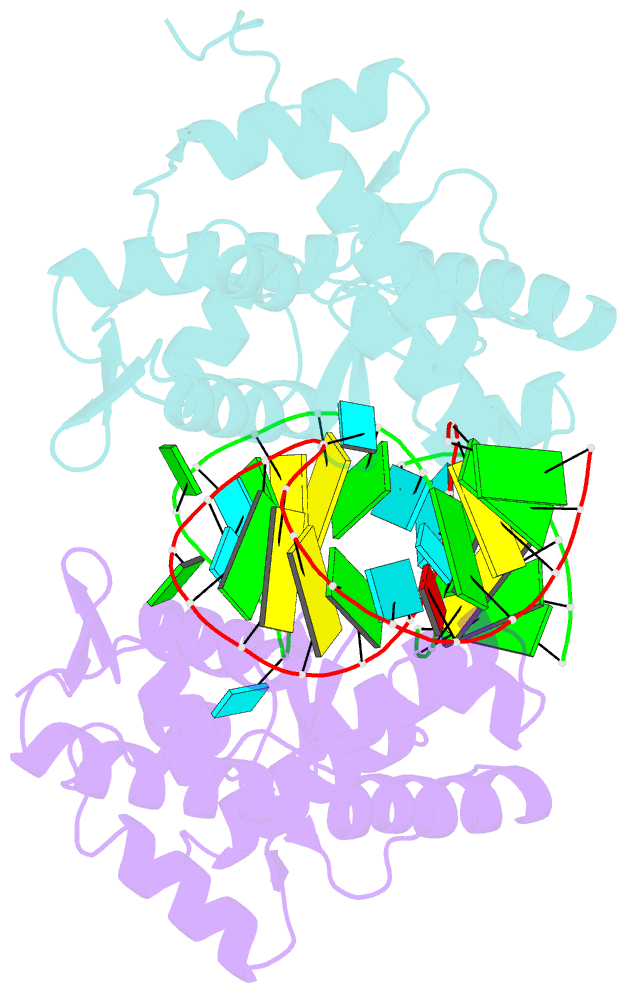
- C-terminal domain(wh2-wh4) of elongation factor selb in complex with secis RNA
- Reference
- Ose T, Soler N, Rasubala L, Kuroki K, Kohda D, Fourmy D, Yoshizawa S, Maenaka K (2007): "Structural Basis for Dynamic Interdomain Movement and RNA Recognition of the Selenocysteine-Specific Elongation Factor Selb." Structure, 15, 577. doi: 10.1016/J.STR.2007.03.007.
- Abstract
- Selenocysteine (Sec) is the "21st" amino acid and is genetically encoded by an unusual incorporation system. The stop codon UGA becomes a Sec codon when the selenocysteine insertion sequence (SECIS) exists downstream of UGA. Sec incorporation requires a specific elongation factor, SelB, which recognizes tRNA(Sec) via use of an EF-Tu-like domain and the SECIS mRNA hairpin via use of a C-terminal domain (SelB-C). SelB functions in multiple translational steps: binding to SECIS mRNA and tRNA(Sec), delivery of tRNA(Sec) onto an A site, GTP hydrolysis, and release from tRNA and mRNA. However, this dynamic mechanism remains to be revealed. Here, we report a large domain rearrangement in the structure of SelB-C complexed with RNA. Surprisingly, the interdomain region forms new interactions with the phosphate backbone of a neighboring RNA, distinct from SECIS RNA binding. This SelB-RNA interaction is sequence independent, possibly reflecting SelB-tRNA/-rRNA recognitions. Based on these data, the dynamic SelB-ribosome-mRNA-tRNA interactions will be discussed.