Summary information and primary citation
- PDB-id
- 2uzk; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- X-ray (2.7 Å)
- Summary
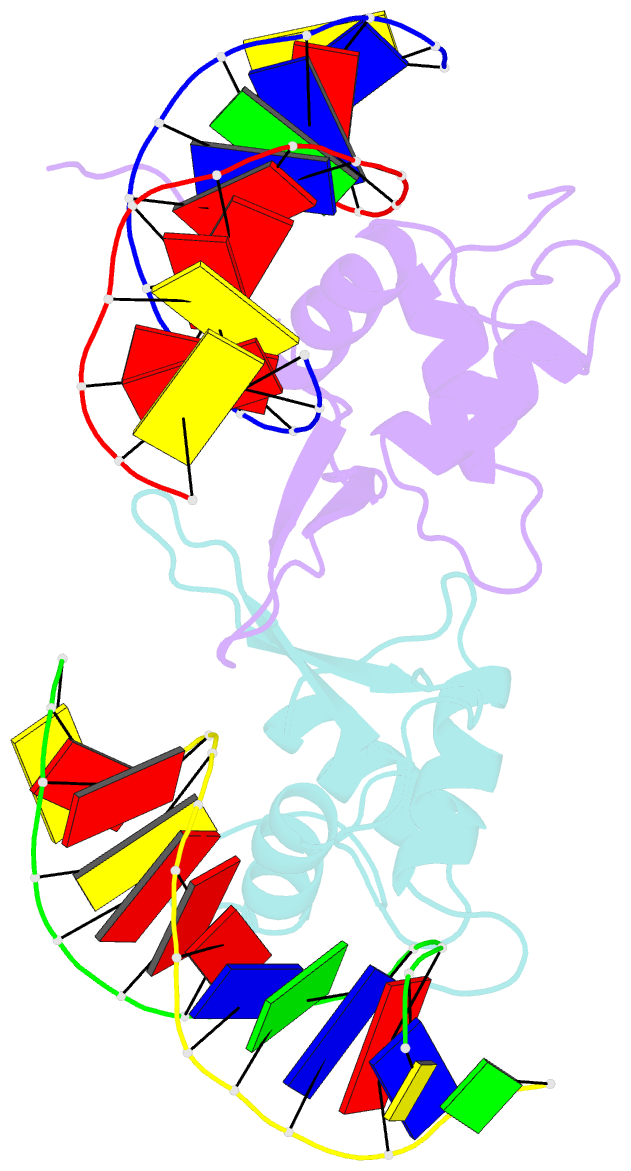
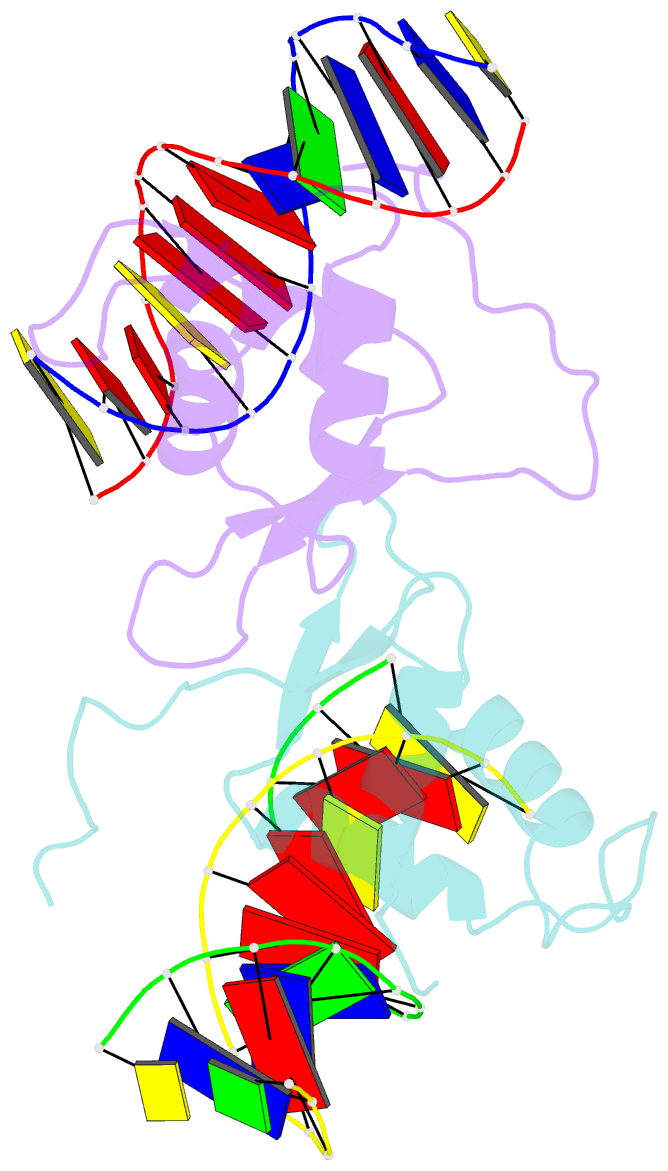
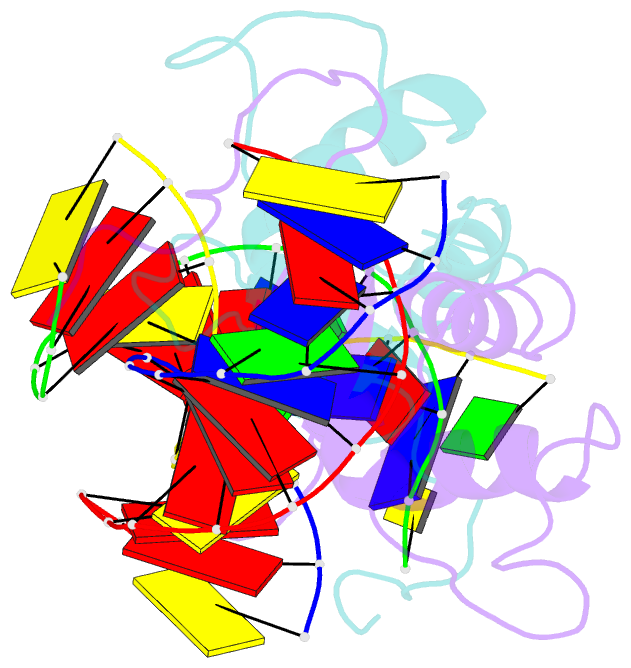
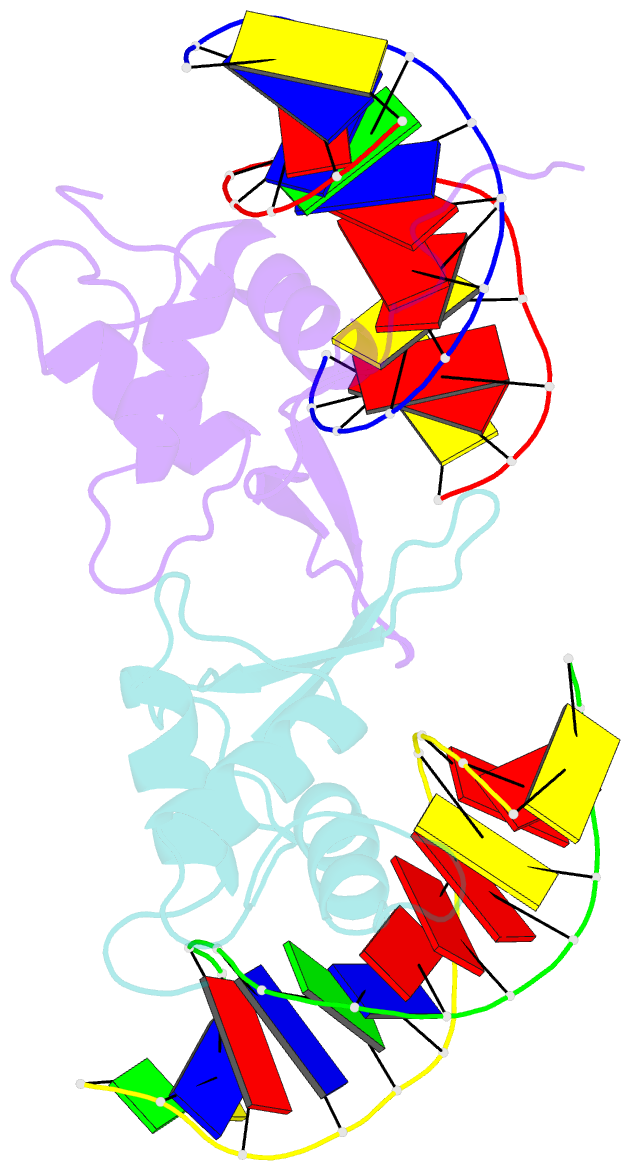
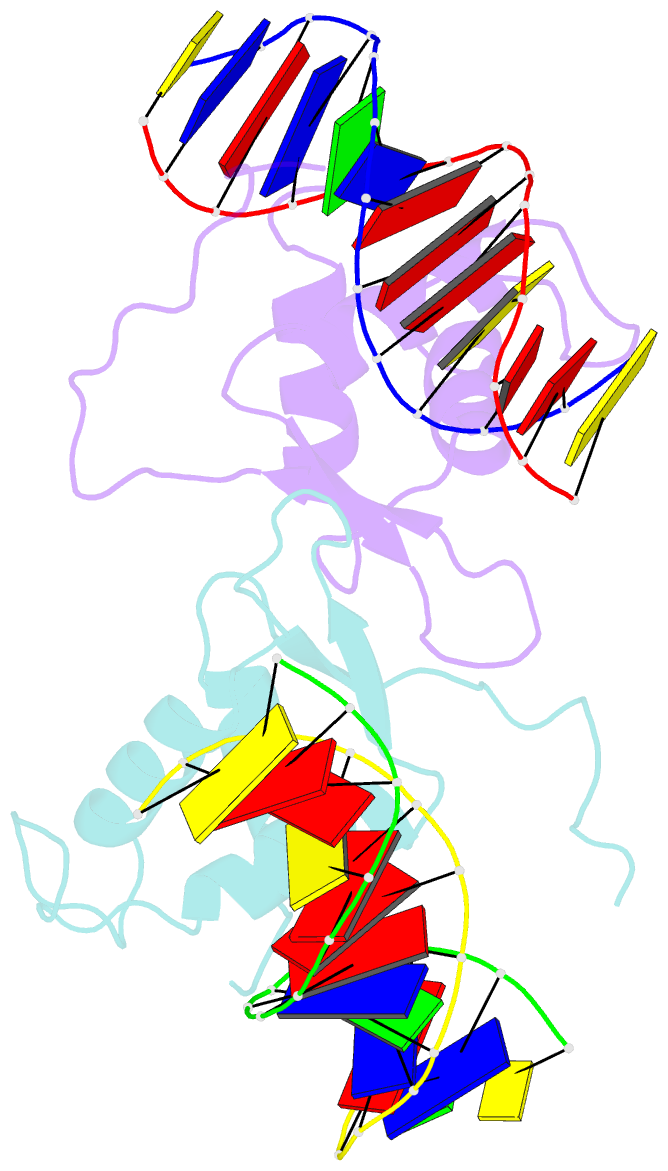
- Crystal structure of the human foxo3a-dbd bound to DNA
- Reference
- Tsai K-L, Sun Y-J, Huang C-Y, Yang J-Y, Hung M-C, Hsiao C-D (2007): "Crystal Structure of the Human Foxo3A-Dbd/DNA Complex Suggests the Effects of Post-Translational Modification." Nucleic Acids Res., 35, 6984. doi: 10.1093/NAR/GKM703.
- Abstract
- FOXO3a is a transcription factor of the FOXO family. The FOXO proteins participate in multiple signaling pathways, and their transcriptional activity is regulated by several post-translational mechanisms, including phosphorylation, acetylation and ubiquitination. Because these post-translational modification sites are located within the C-terminal basic region of the FOXO DNA-binding domain (FOXO-DBD), it is possible that these post-translational modifications could alter the DNA-binding characteristics. To understand how FOXO mediate transcriptional activity, we report here the 2.7 A crystal structure of the DNA-binding domain of FOXO3a (FOXO3a-DBD) bound to a 13-bp DNA duplex containing a FOXO consensus binding sequence (GTAAACA). Based on a unique structural feature in the C-terminal region and results from biochemical and mutational studies, our studies may explain how FOXO-DBD C-terminal phosphorylation by protein kinase B (PKB) or acetylation by cAMP-response element binding protein (CBP) can attenuate the DNA-binding activity and thereby reduce transcriptional activity of FOXO proteins. In addition, we demonstrate that the methyl groups of specific thymine bases within the consensus sequence are important for FOXO3a-DBD recognition of the consensus binding site.