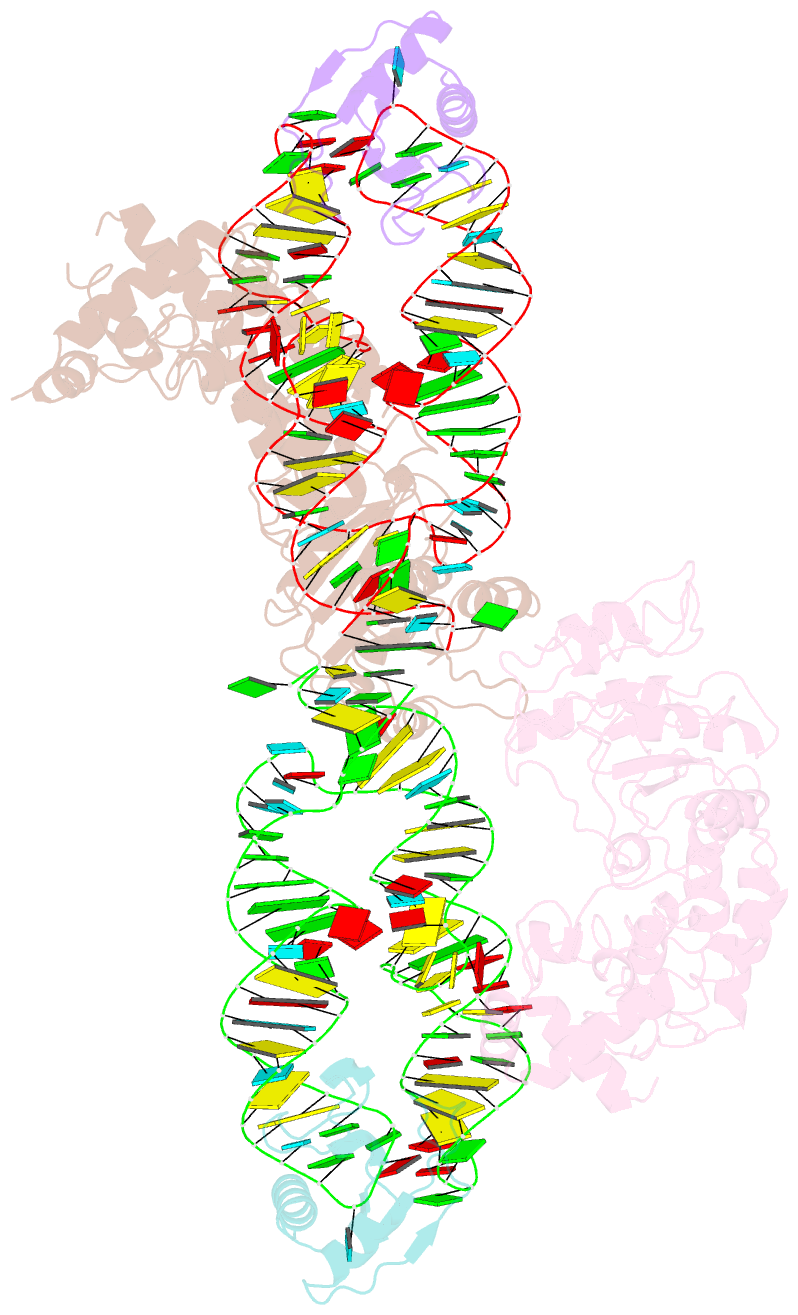
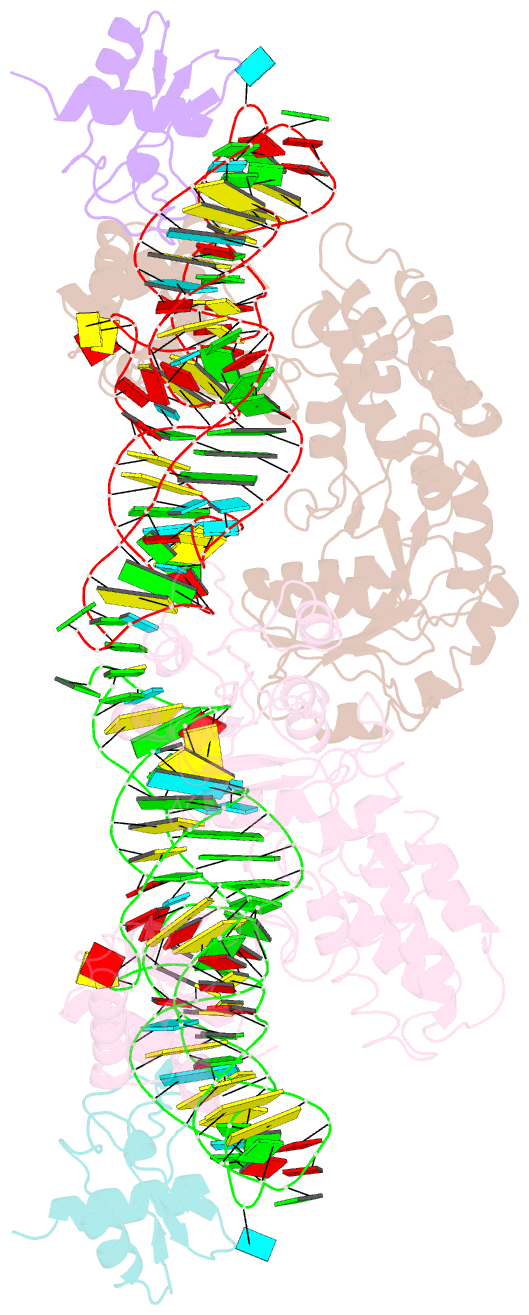
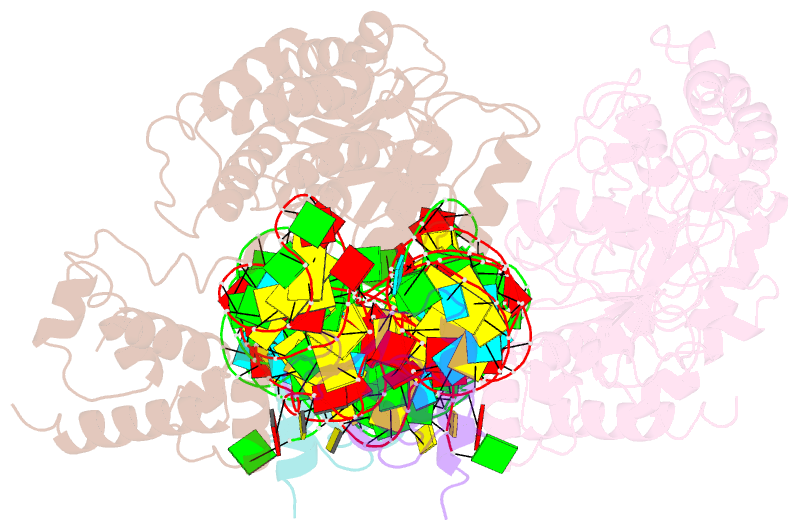
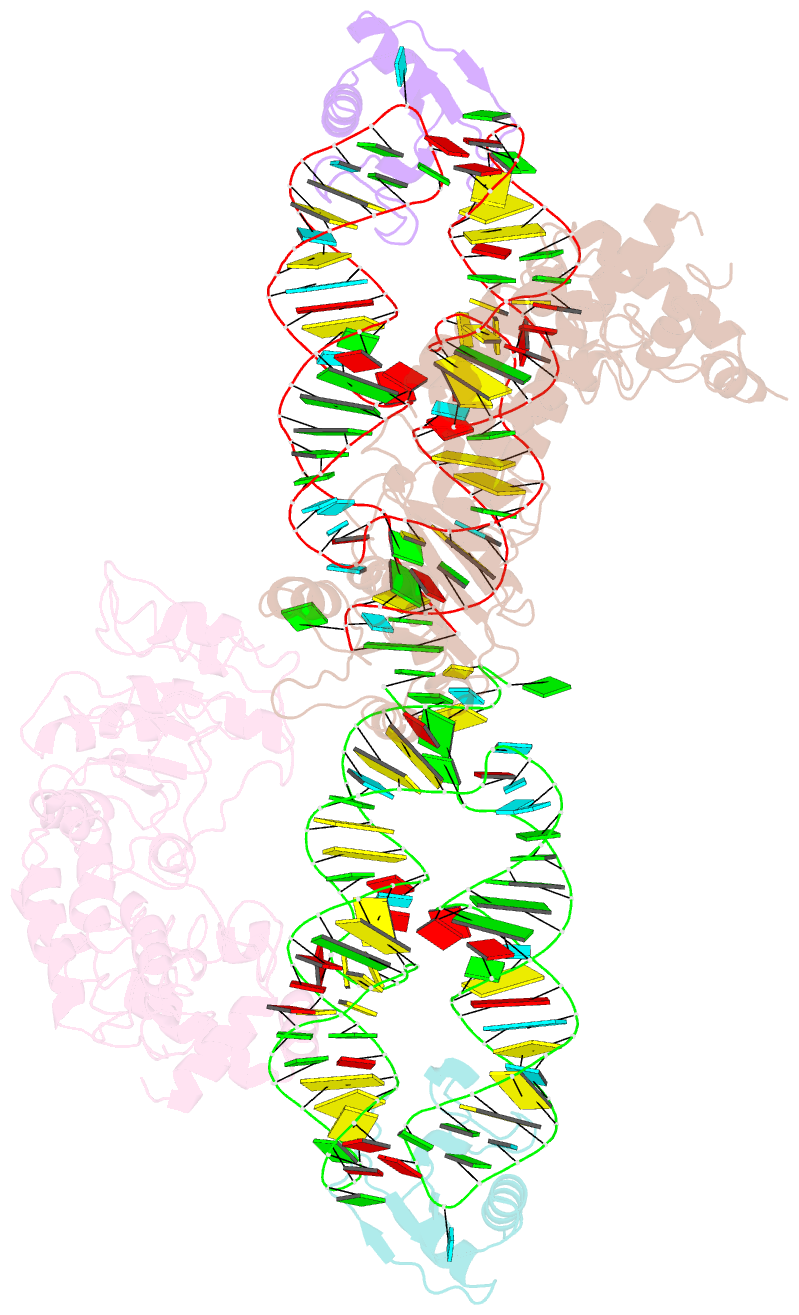
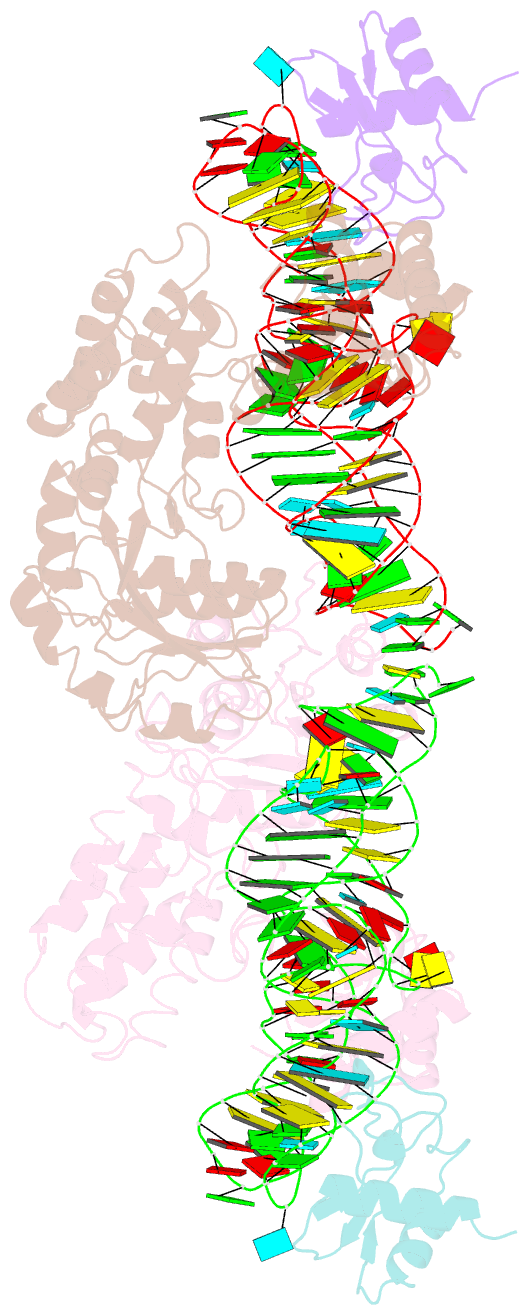
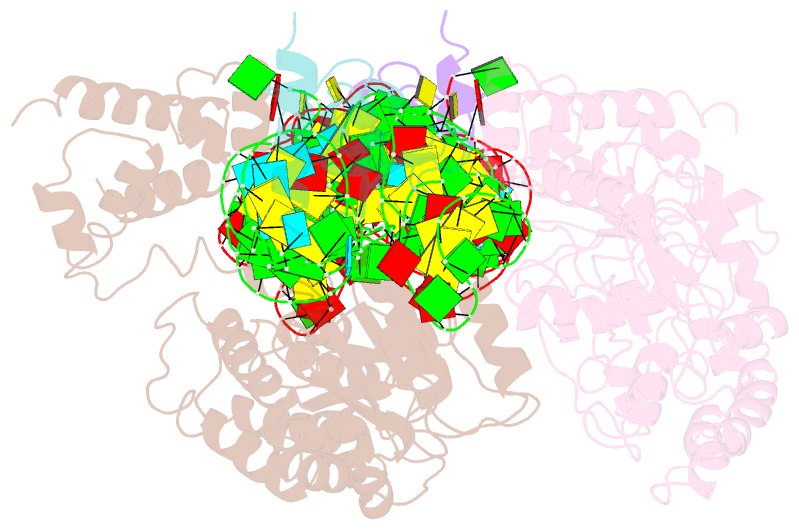
Summary information and primary citation
- PDB-id
- 2v3c; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- signaling protein
- Method
- X-ray (2.5 Å)
- Summary
- Crystal structure of the srp54-srp19-7s.s srp RNA complex of m. jannaschii
- Reference
- Hainzl T, Huang S, Sauer-Eriksson AE (2007): "Interaction of Signal-Recognition Particle 54 Gtpase Domain and Signal-Recognition Particle RNA in the Free Signal-Recognition Particle." Proc.Natl.Acad.Sci.USA, 104, 14911. doi: 10.1073/PNAS.0702467104.
- Abstract
- The signal-recognition particle (SRP) is a ubiquitous protein-RNA complex that targets proteins to cellular membranes for insertion or secretion. A key player in SRP-mediated protein targeting is the evolutionarily conserved core consisting of the SRP RNA and the multidomain protein SRP54. Communication between the SRP54 domains is critical for SRP function, where signal sequence binding at the M domain directs receptor binding at the GTPase domain (NG domain). These SRP activities are linked to domain rearrangements, for which the role of SRP RNA is not clear. In free SRP, a direct interaction of the GTPase domain with SRP RNA has been proposed but has never been structurally verified. In this study, we present the crystal structure at 2.5-A resolution of the SRP54-SRP19-SRP RNA complex of Methanococcus jannaschii SRP. The structure reveals an RNA-bound conformation of the SRP54 GTPase domain, in which the domain is spatially well separated from the signal peptide binding site. The association of both the N and G domains with SRP RNA in free SRP provides further structural evidence for the pivotal role of SRP RNA in the regulation of the SRP54 activity.