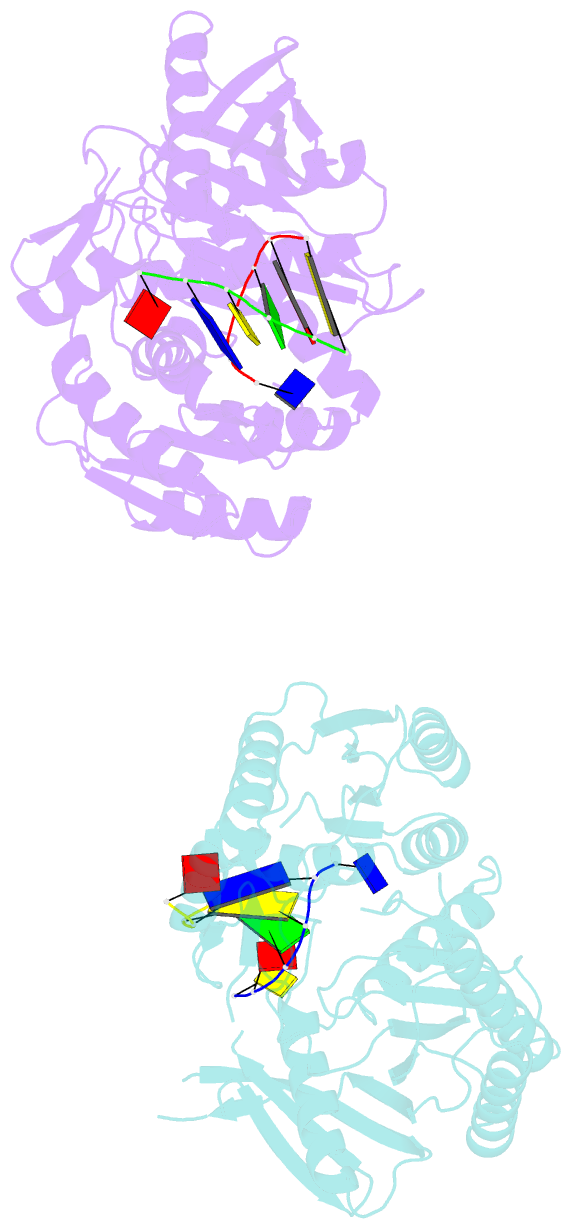
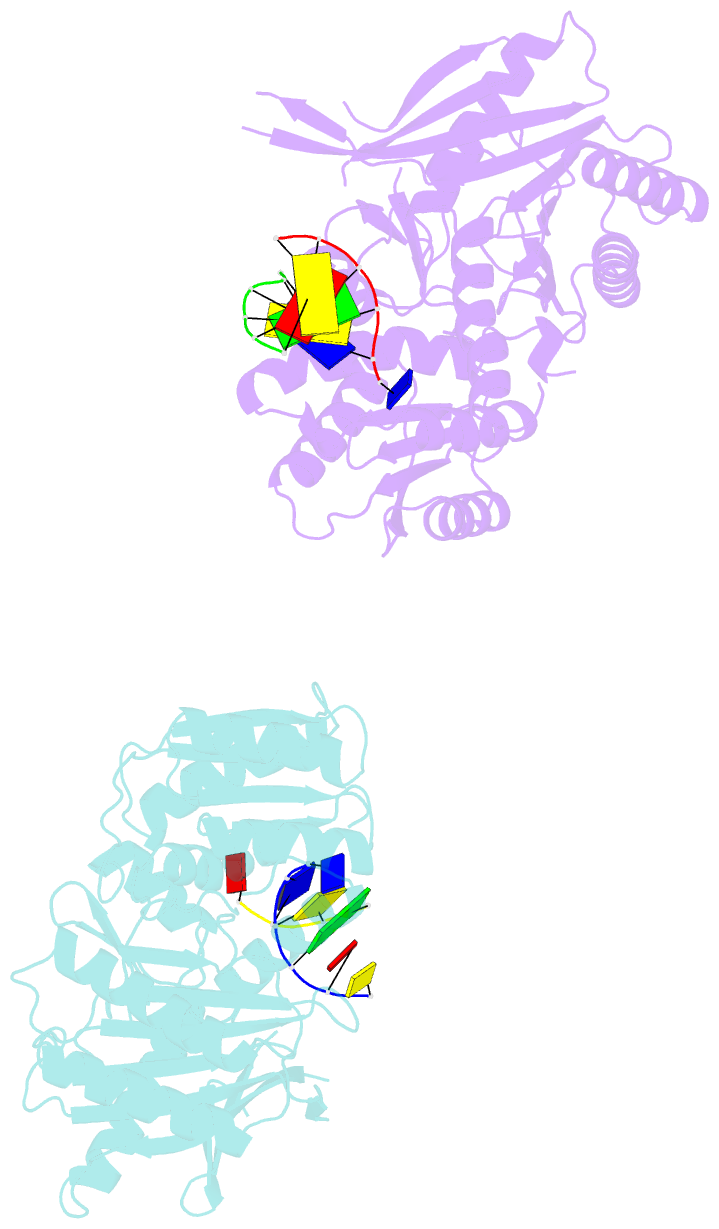
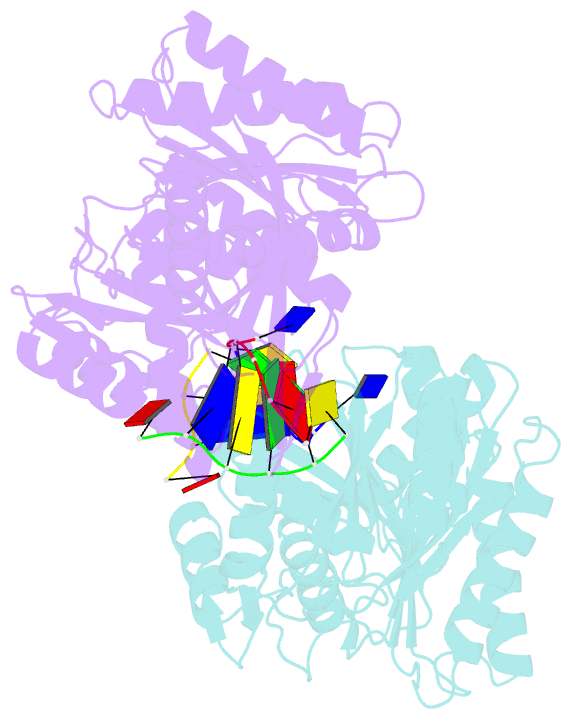
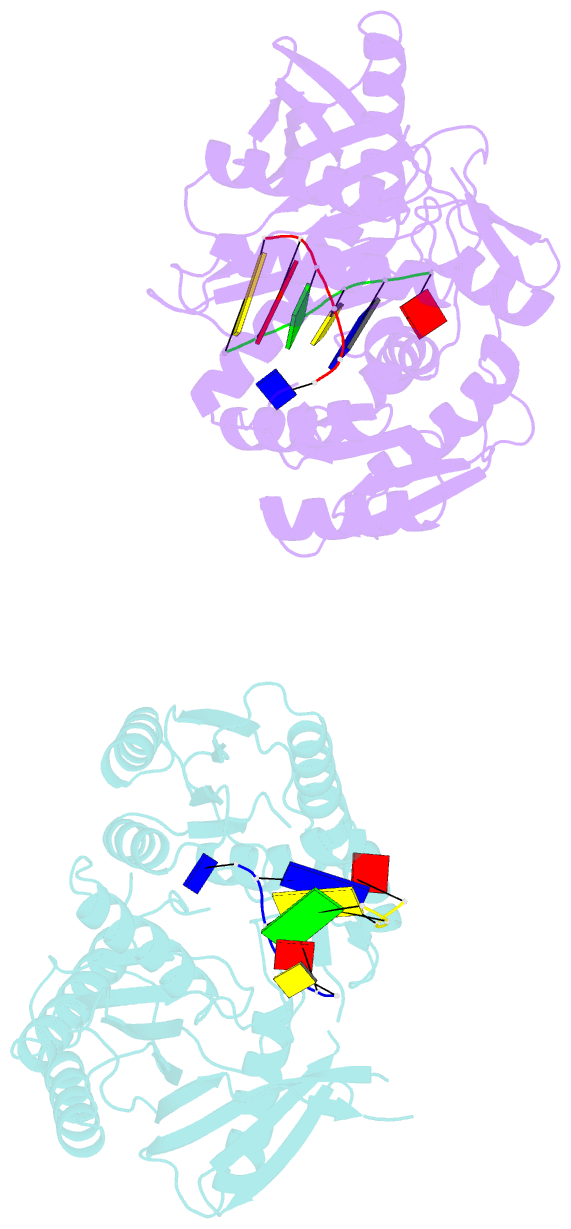
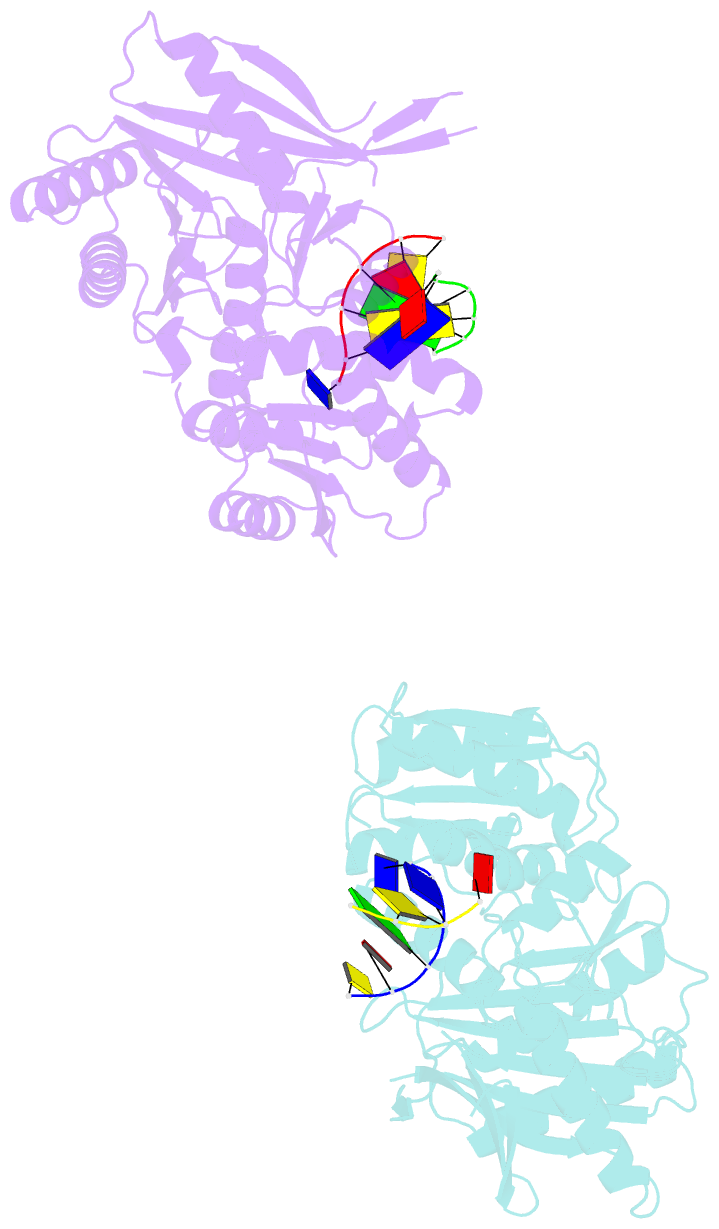
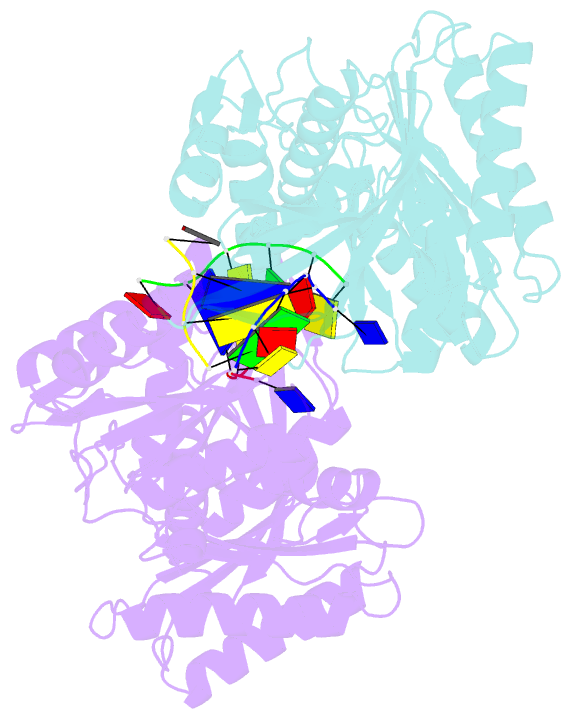
Summary information and primary citation
- PDB-id
- 2w42; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- protein-DNA complex
- Method
- X-ray (1.9 Å)
- Summary
- The structure of a piwi protein from archaeoglobus fulgidus complexed with a 16nt DNA duplex.
- Reference
- Parker JS, Parizotto EA, Wang M, Roe SM, Barford D (2009): "Enhancement of the Seed-Target Recognition Step in RNA Silencing by a Piwi-Mid Domain Protein." Mol.Cell, 33, 204. doi: 10.1016/J.MOLCEL.2008.12.012.
- Abstract
- Target recognition in RNA silencing is governed by the "seed sequence" of a guide RNA strand associated with the PIWI/MID domain of an Argonaute protein in RISC. Using a reconstituted in vitro target recognition system, we show that a model PIWI/MID domain protein confers position-dependent tightening and loosening of guide-strand-target interactions. Over the seed sequence, the interaction affinity is enhanced up to approximately 300-fold. Enhancement is achieved through a reduced entropy penalty for the interaction. In contrast, interactions 3' of the seed are inhibited. We quantified mismatched target recognition inside and outside the seed, revealing amplified discrimination at the third position in the seed mediated by the PIWI/MID domain. Thus, association of the guide strand with the PIWI/MID domain generates an enhanced affinity anchor site over the seed that can promote fidelity in target recognition and stabilize and guide the assembly of the active silencing complex.