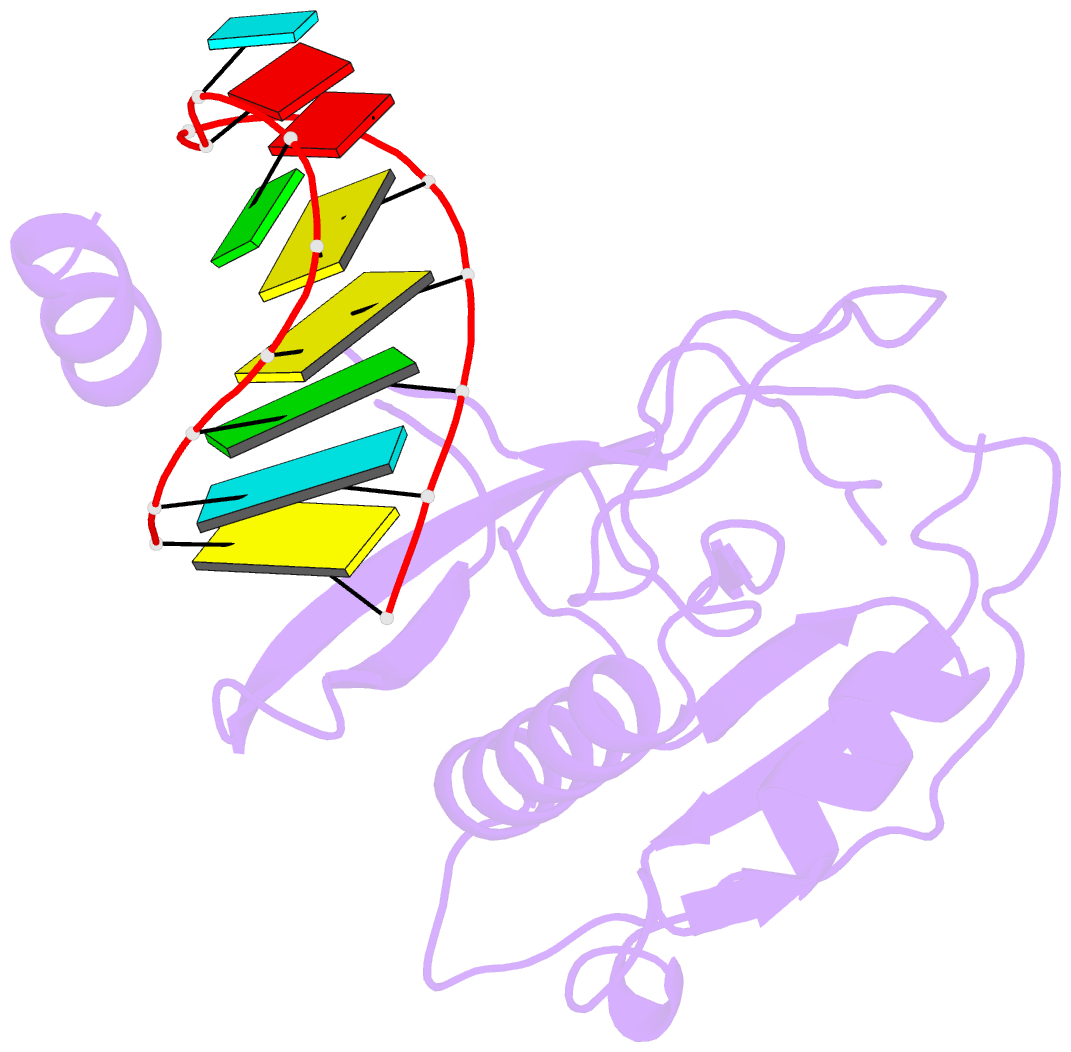
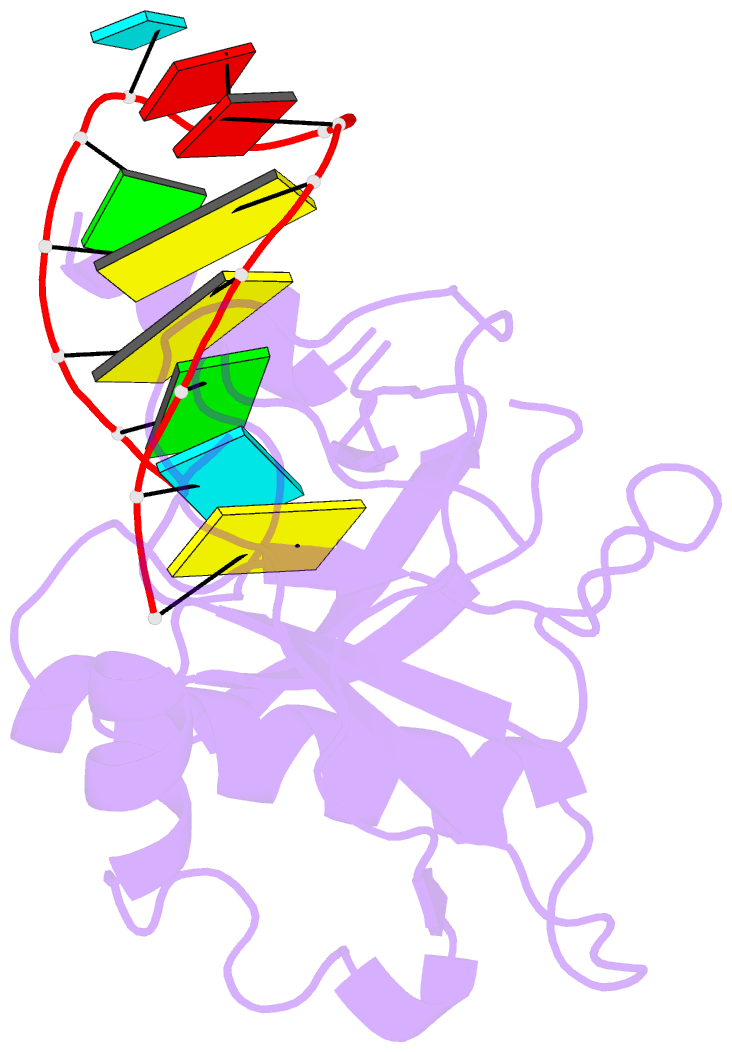
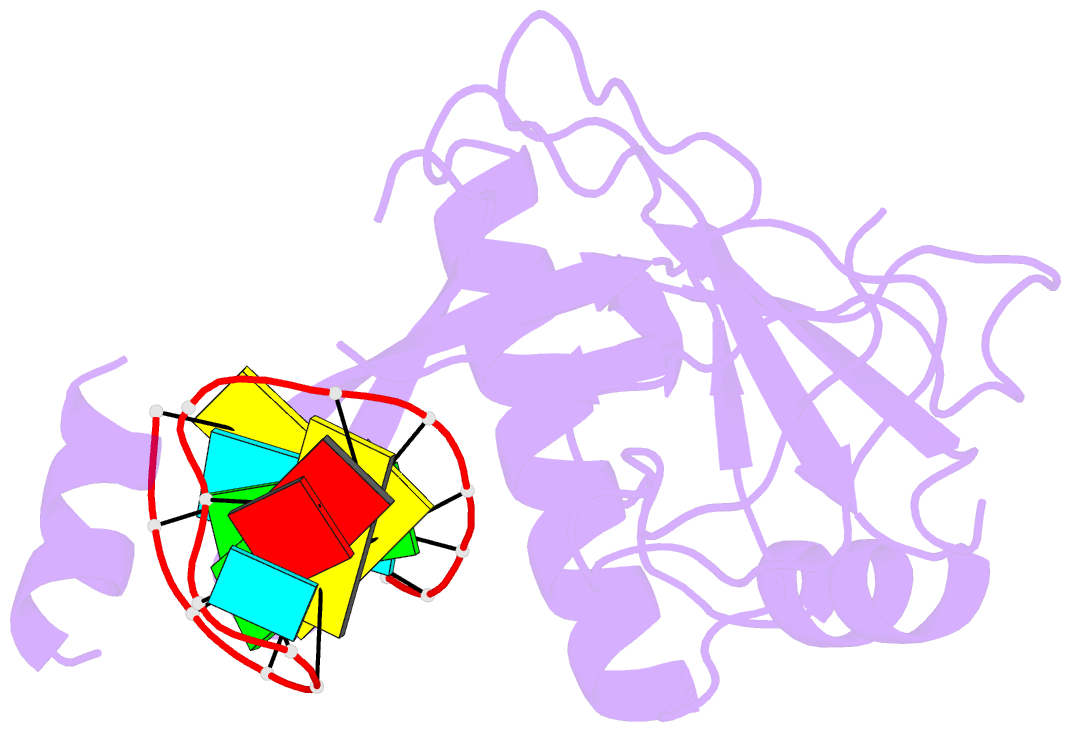
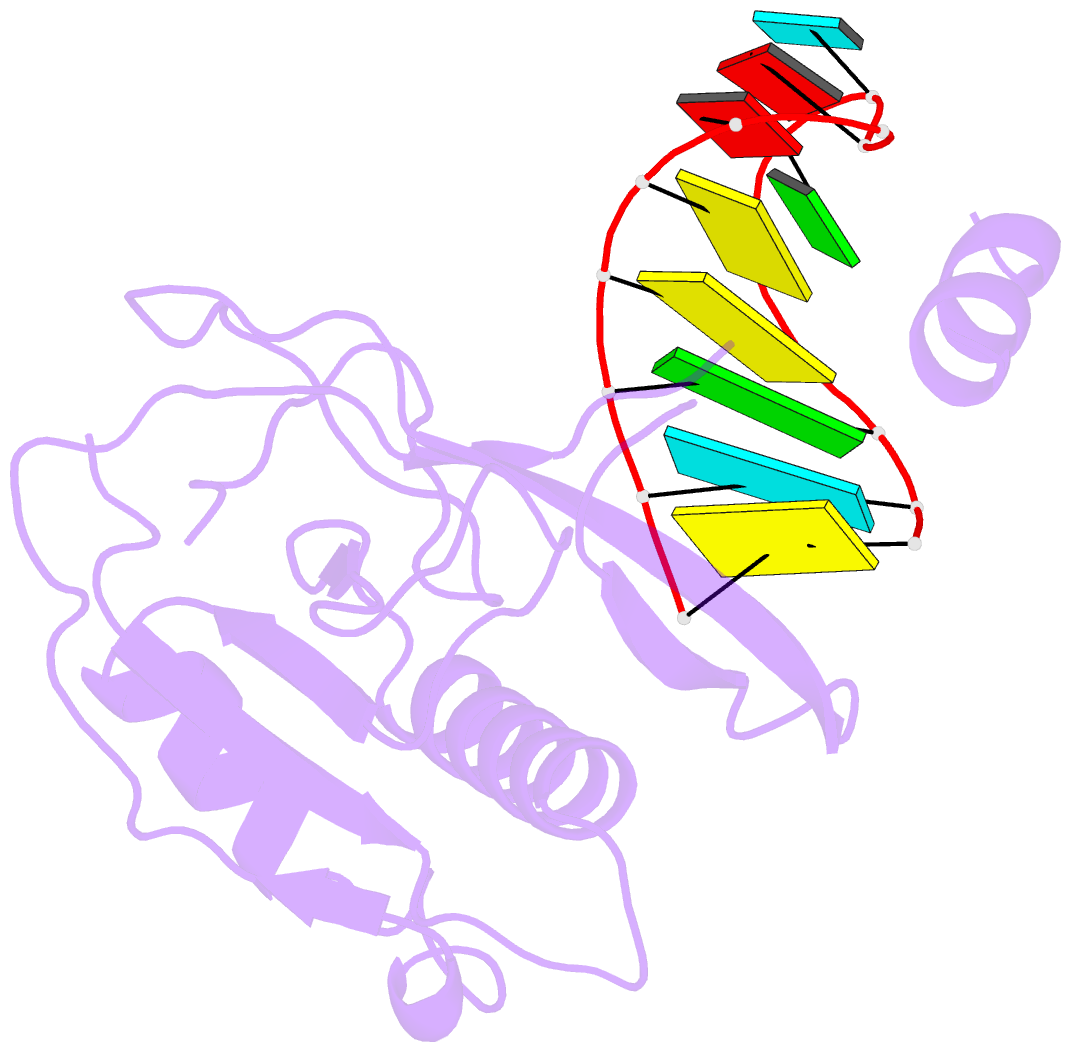
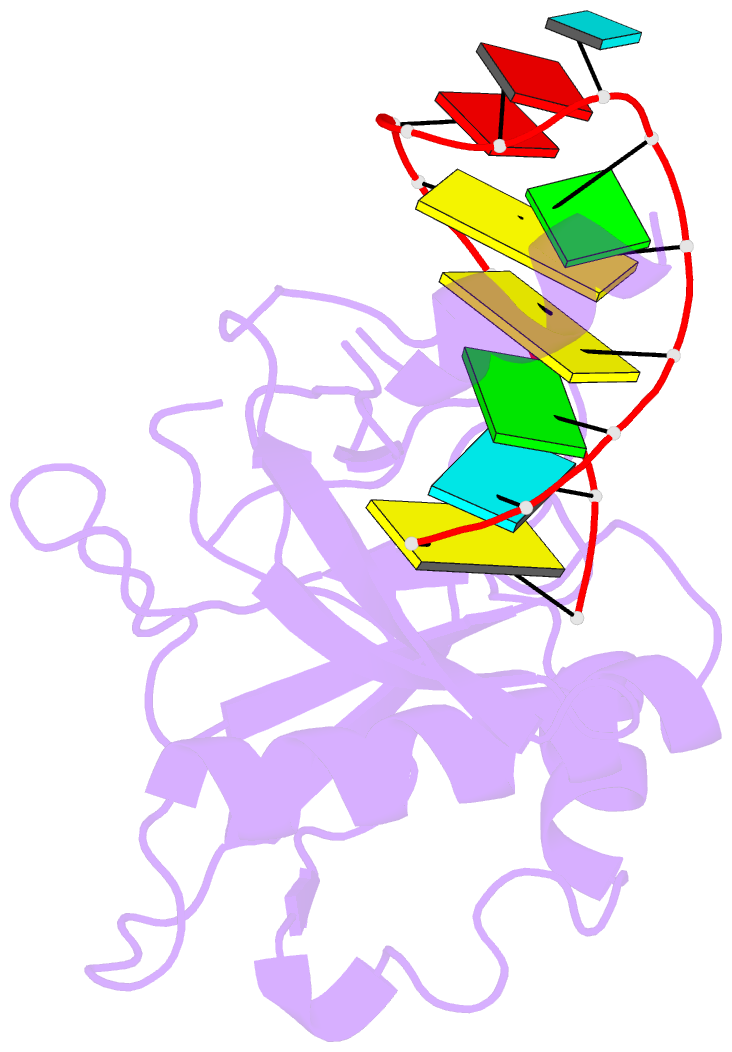
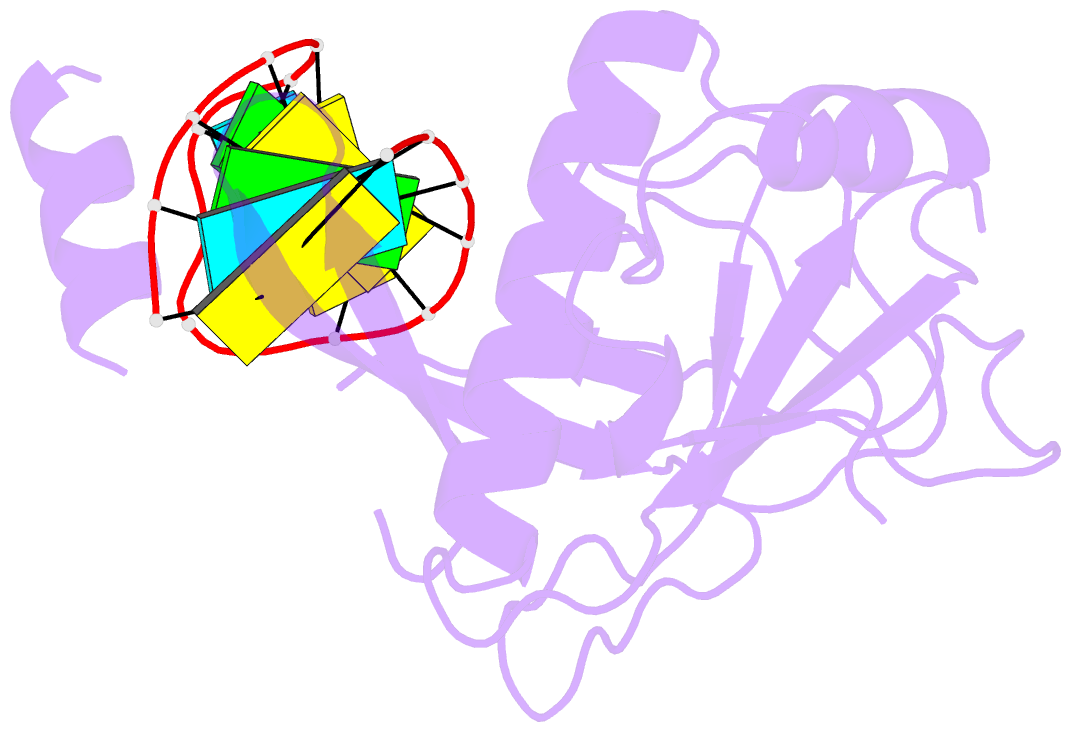
Summary information and primary citation
- PDB-id
- 2xli; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA
- Method
- X-ray (2.33 Å)
- Summary
- Crystal structure of the csy4-crrna complex, monoclinic form
- Reference
- Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA (2010): "Sequence- and Structure-Specific RNA Processing by a Crispr Endonuclease." Science, 329, 1355. doi: 10.1126/SCIENCE.1192272.
- Abstract
- Many bacteria and archaea contain clustered regularly interspaced short palindromic repeats (CRISPRs) that confer resistance to invasive genetic elements. Central to this immune system is the production of CRISPR-derived RNAs (crRNAs) after transcription of the CRISPR locus. Here, we identify the endoribonuclease (Csy4) responsible for CRISPR transcript (pre-crRNA) processing in Pseudomonas aeruginosa. A 1.8 angstrom crystal structure of Csy4 bound to its cognate RNA reveals that Csy4 makes sequence-specific interactions in the major groove of the crRNA repeat stem-loop. Together with electrostatic contacts to the phosphate backbone, these enable Csy4 to bind selectively and cleave pre-crRNAs using phylogenetically conserved serine and histidine residues in the active site. The RNA recognition mechanism identified here explains sequence- and structure-specific processing by a large family of CRISPR-specific endoribonucleases.