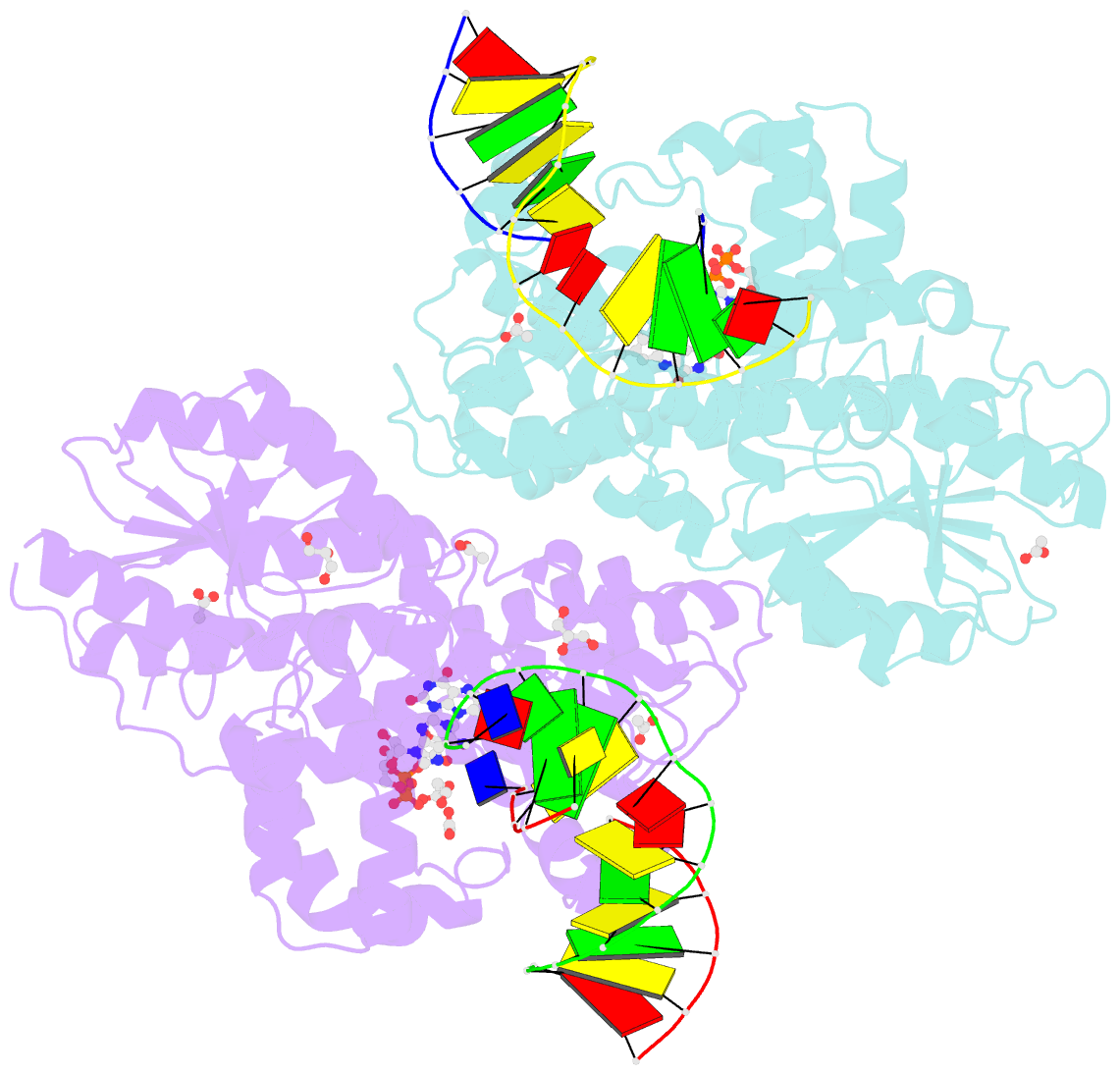
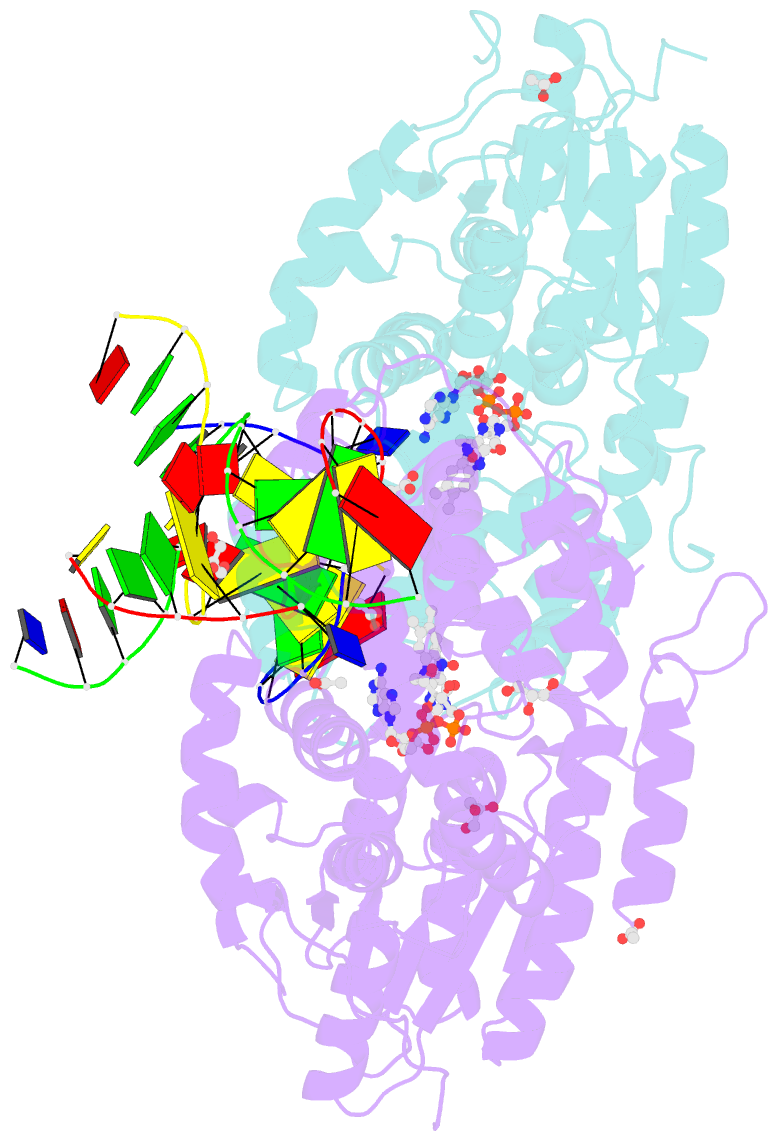
Summary information and primary citation
- PDB-id
- 2xrz; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- lyase-DNA
- Method
- X-ray (2.2 Å)
- Summary
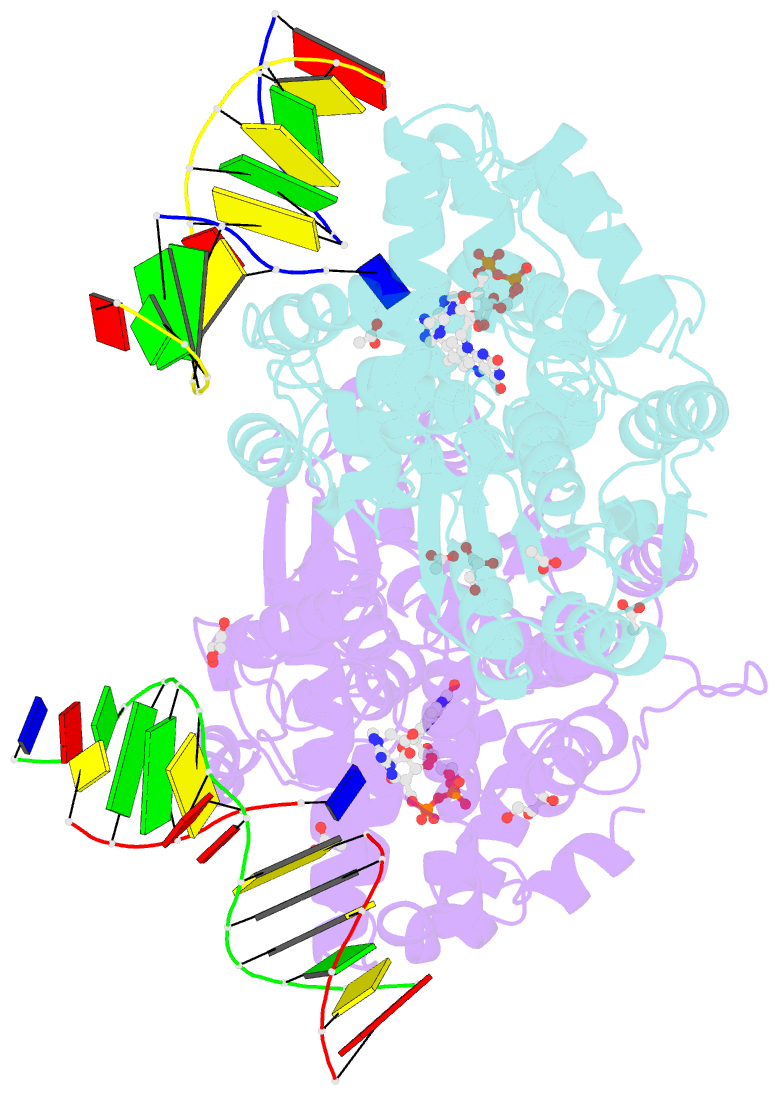
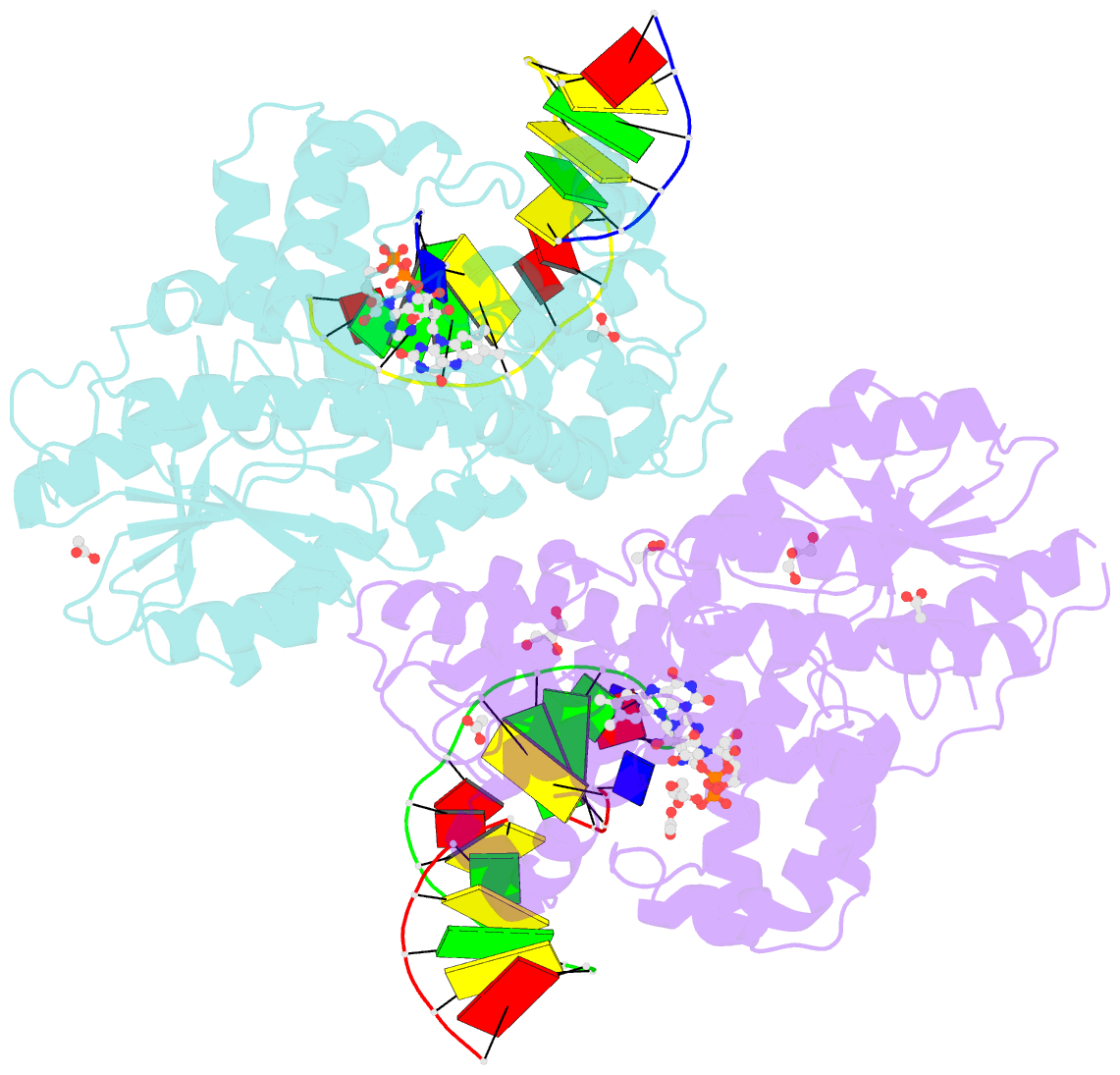
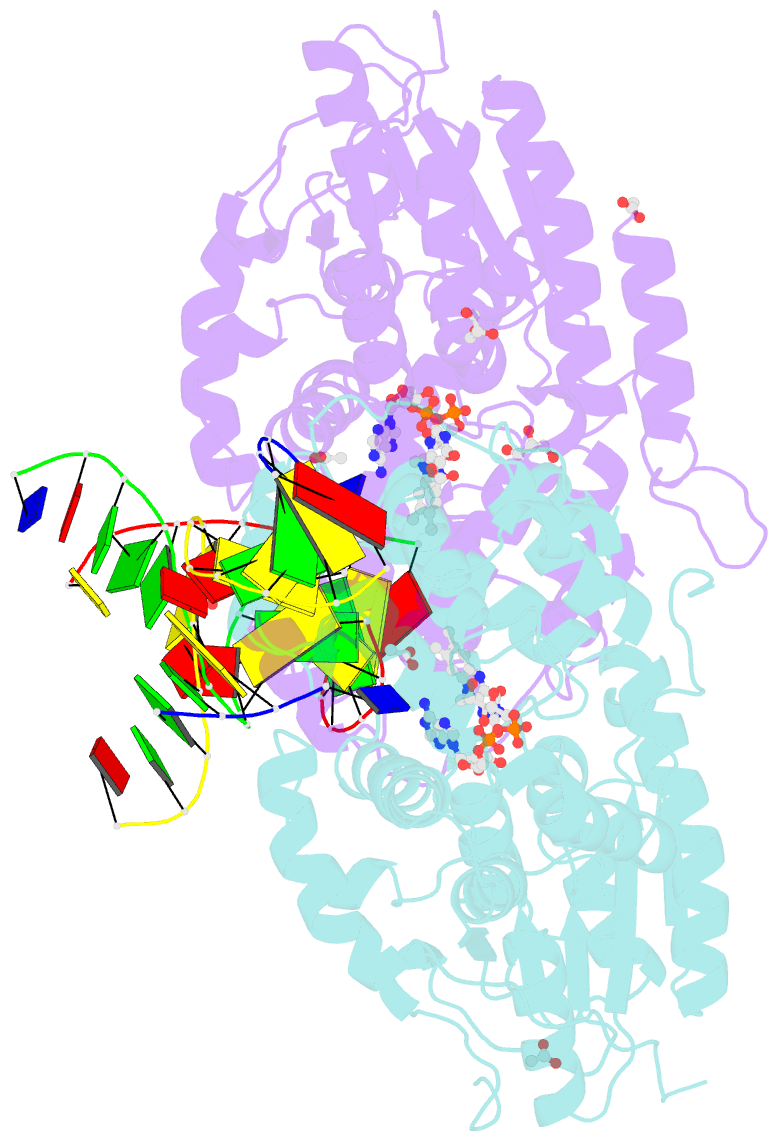
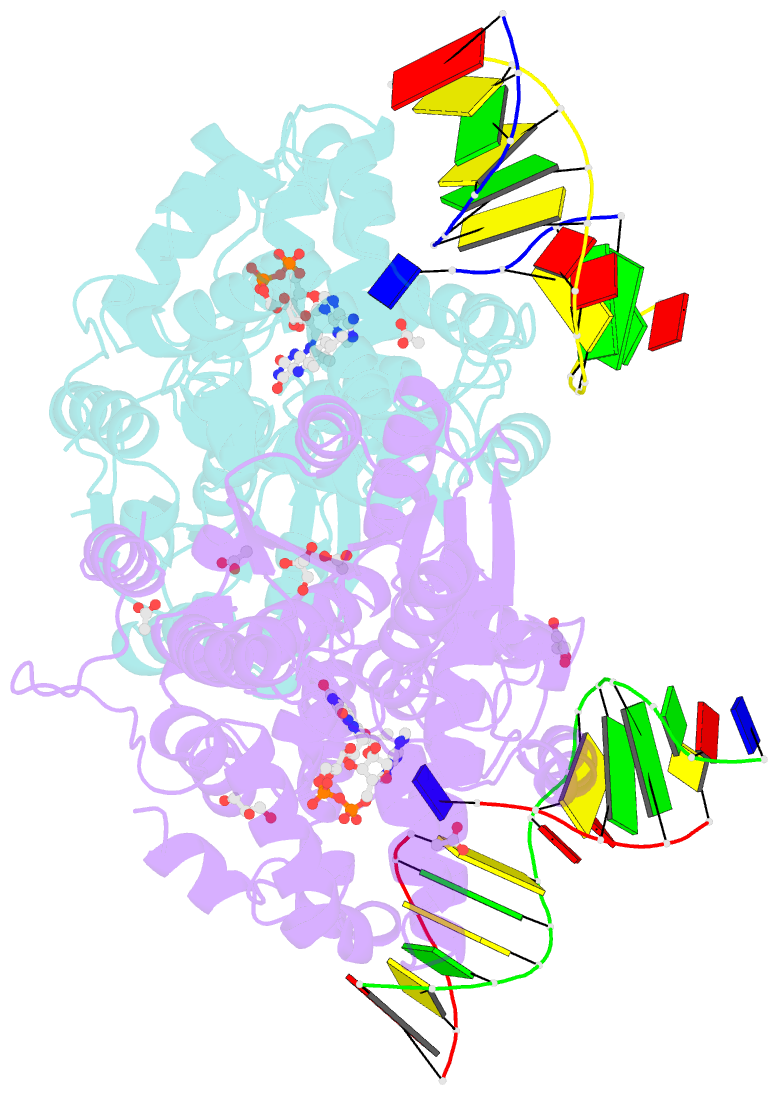
- X-ray structure of archaeal class ii cpd photolyase from methanosarcina mazei in complex with intact cpd-lesion
- Reference
- Kiontke S, Geisselbrecht Y, Pokorny R, Carell T, Batschauer A, Essen LO (2011): "Crystal Structures of an Archaeal Class II DNA Photolyase and its Complex with Uv-Damaged Duplex DNA." Embo J., 30, 4437. doi: 10.1038/EMBOJ.2011.313.
- Abstract
- Class II photolyases ubiquitously occur in plants, animals, prokaryotes and some viruses. Like the distantly related microbial class I photolyases, these enzymes repair UV-induced cyclobutane pyrimidine dimer (CPD) lesions within duplex DNA using blue/near-UV light. Methanosarcina mazei Mm0852 is a class II photolyase of the archaeal order of Methanosarcinales, and is closely related to plant and metazoan counterparts. Mm0852 catalyses light-driven DNA repair and photoreduction, but in contrast to class I enzymes lacks a high degree of binding discrimination between UV-damaged and intact duplex DNA. We solved crystal structures of Mm0852, the first one for a class II photolyase, alone and in complex with CPD lesion-containing duplex DNA. The lesion-binding mode differs from other photolyases by a larger DNA-binding site, and an unrepaired CPD lesion is found flipped into the active site and recognized by a cluster of five water molecules next to the bound 3'-thymine base. Different from other members of the photolyase-cryptochrome family, class II photolyases appear to utilize an unusual, conserved tryptophane dyad as electron transfer pathway to the catalytic FAD cofactor.