Summary information and primary citation
- PDB-id
- 2z9o; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- replication-DNA
- Method
- X-ray (3.14 Å)
- Summary
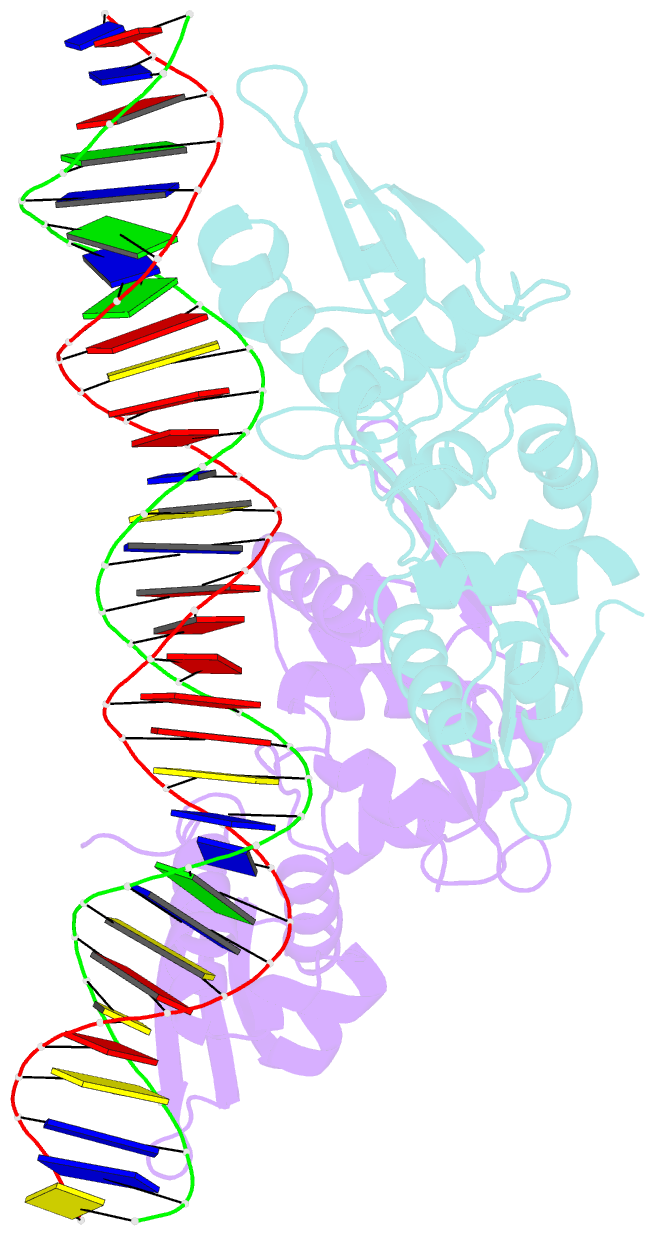
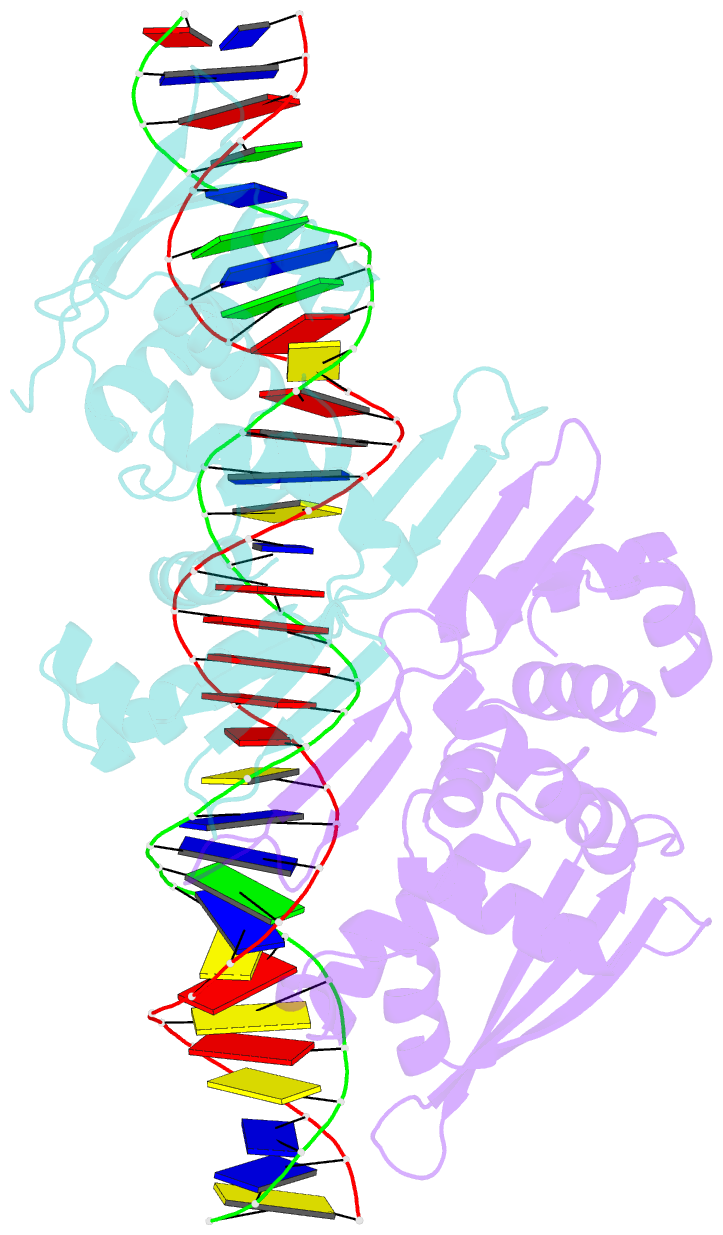
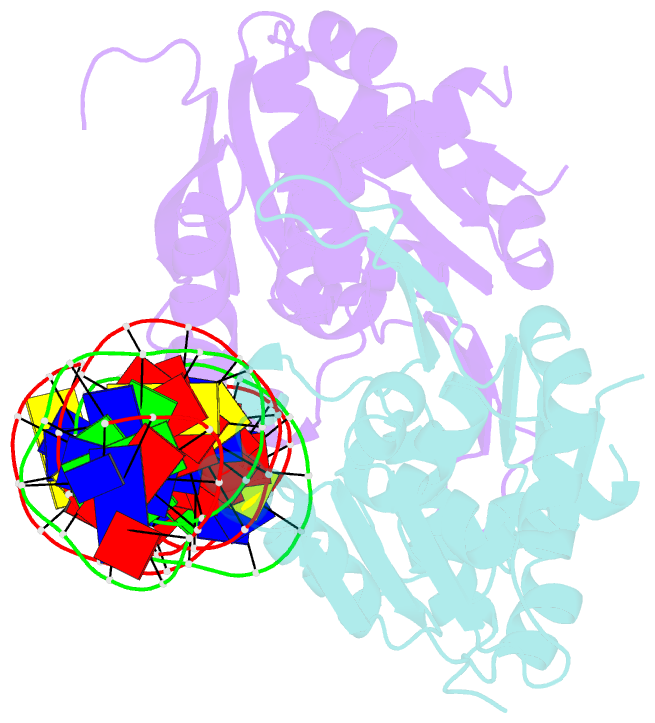
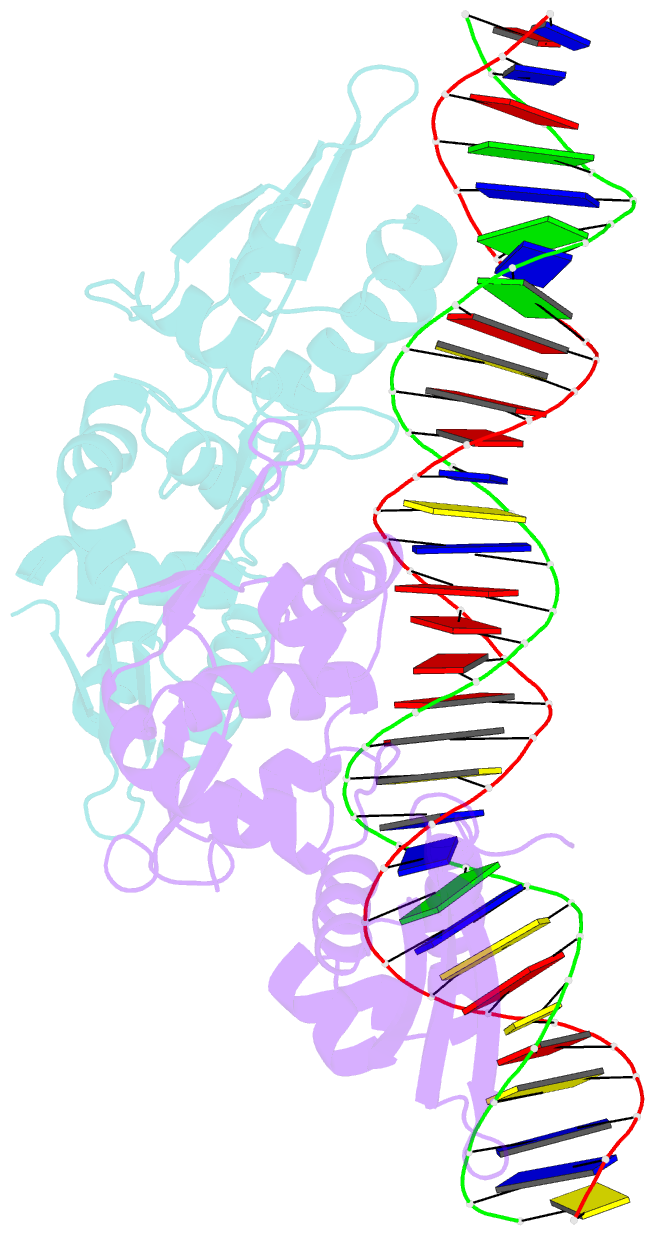
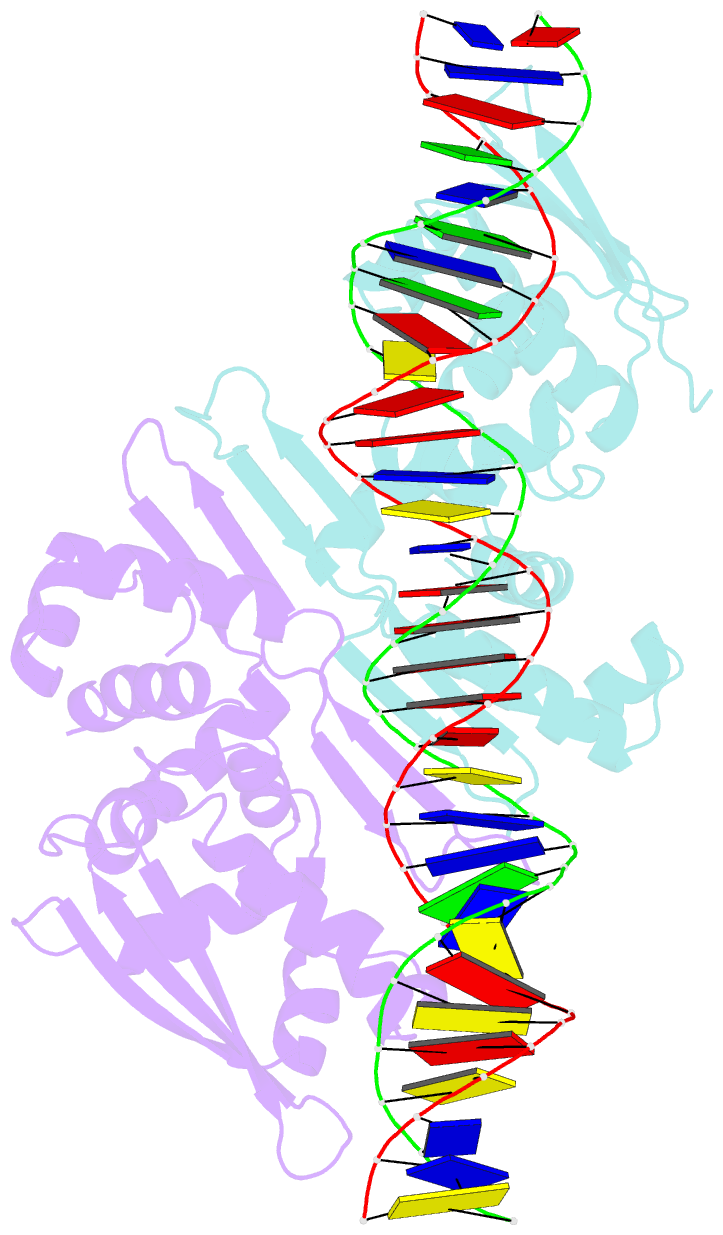
- Crystal structure of the dimeric form of repe in complex with the repe operator DNA
- Reference
- Nakamura A, Wada C, Miki K (2007): "Structural basis for regulation of bifunctional roles in replication initiator protein." Proc.Natl.Acad.Sci.Usa, 104, 18484-18489. doi: 10.1073/pnas.0705623104.
- Abstract
- DNA replication initiator protein RepE stringently regulates F plasmid replication by its two distinct molecular association states. A predominant dimer functions as an autogenous repressor, whereas monomers act as replication initiators, and the dimer requires actions of the DnaK molecular chaperone system for monomerization. The structure of the monomeric form is known, whereas the dimeric structure and structural details of the dimer-to-monomer conversion have been unclear. Here we present the crystal structure of the RepE dimer in complex with the repE operator DNA. The dimerization interface is mainly formed by intermolecular beta-sheets with several key interactions of charged residues. The conformations of the internal N- and C-terminal domains are conserved between the dimer and monomer, whereas the relative domain orientations are strikingly different, allowing for an efficient oligomeric transition of dual-functional RepE. This domain relocation accompanies secondary structural changes in the linker connecting the two domains, and the linker is included in plausible DnaK/DnaJ-binding regions. These findings suggest an activation mechanism for F plasmid replication by RepE monomerization, which is induced and mediated by the DnaK system.