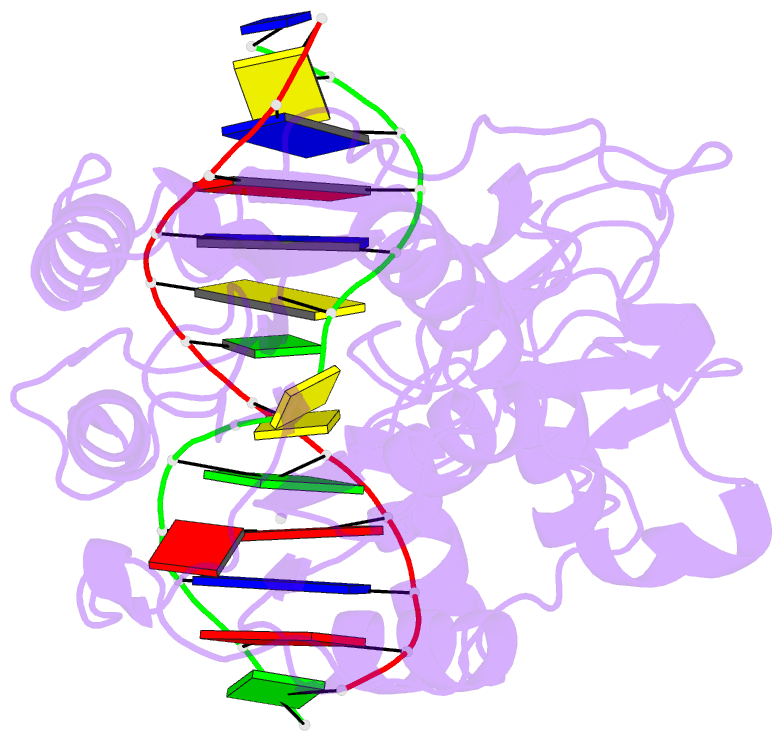
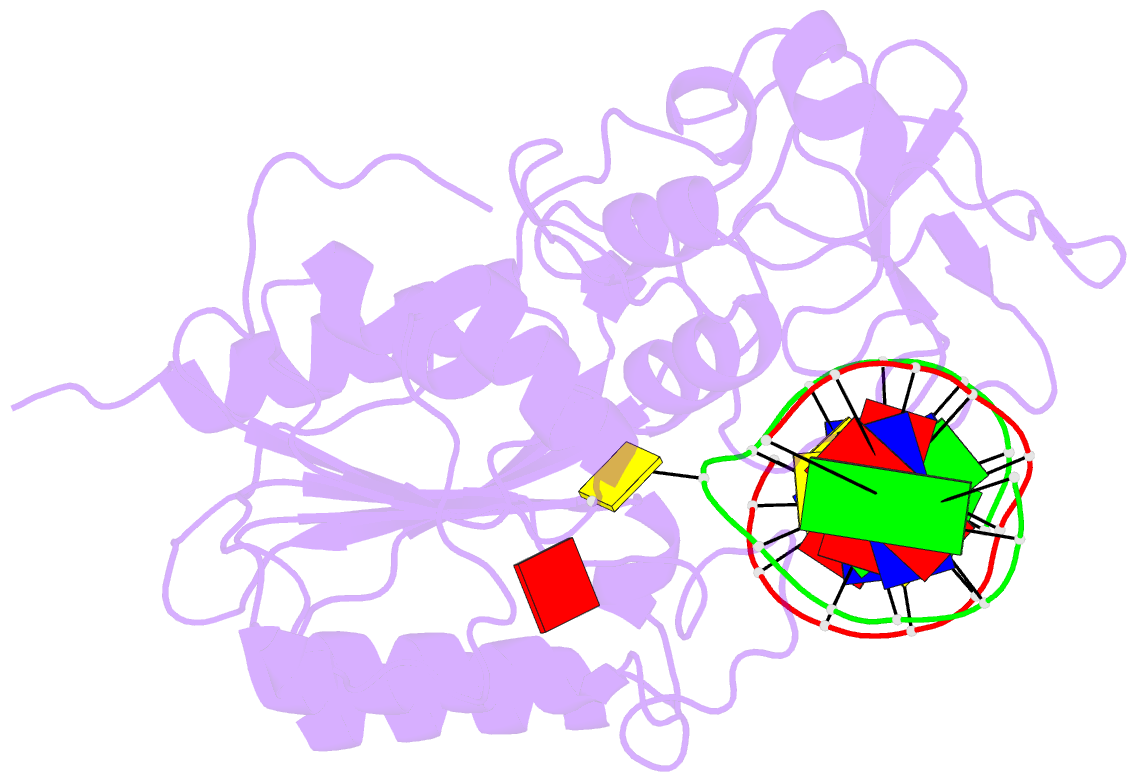
Summary information and primary citation
- PDB-id
- 2zcj; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.75 Å)
- Summary
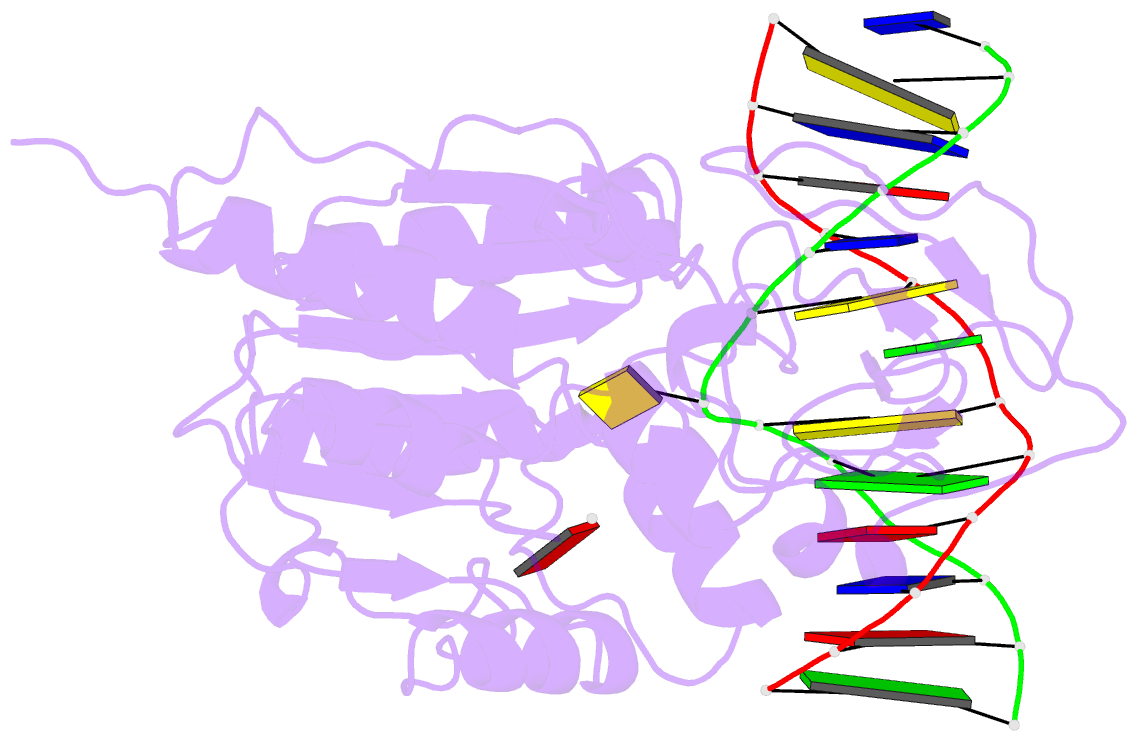
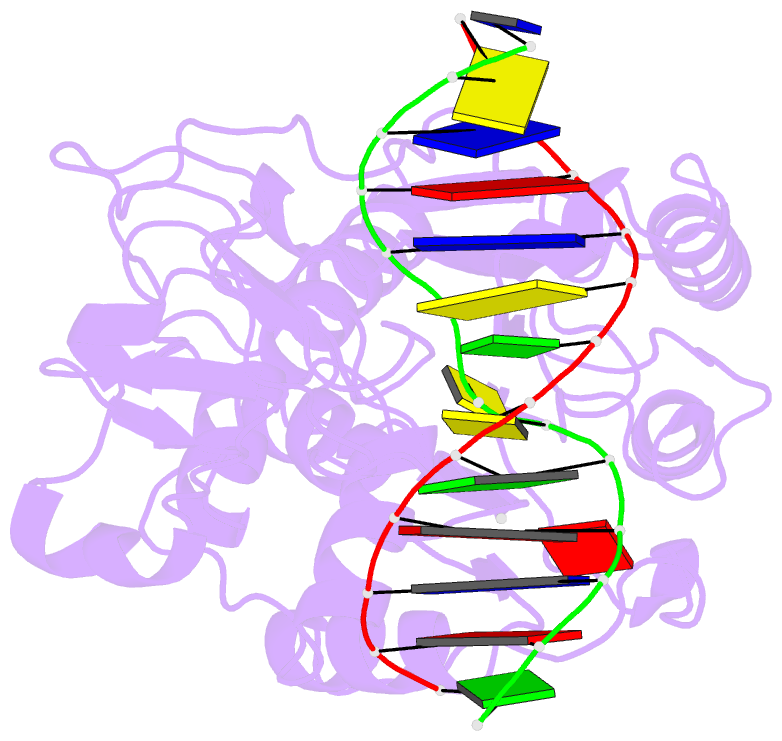
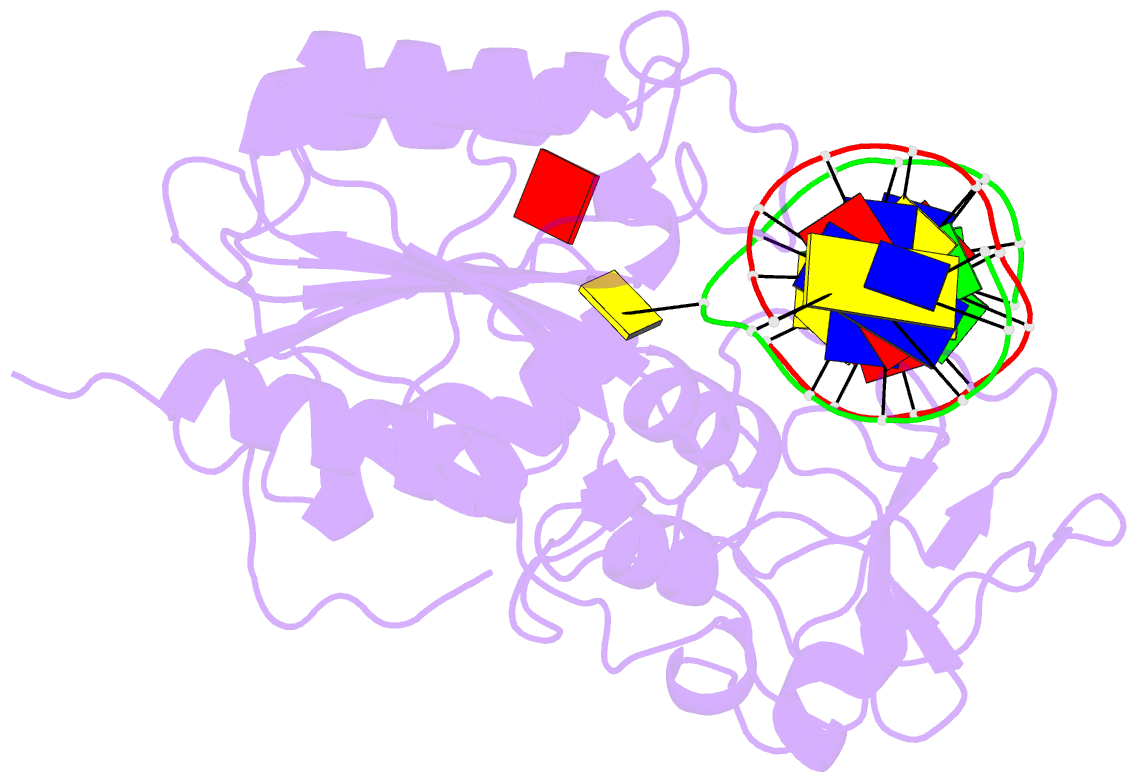
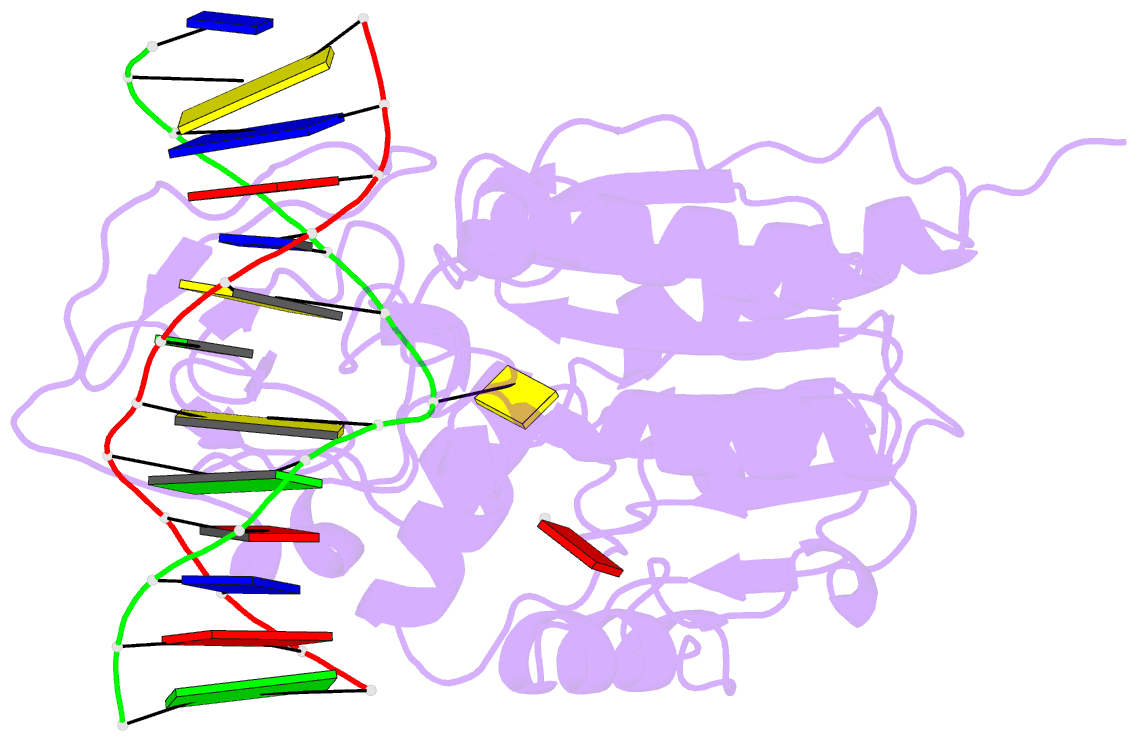
- Ternary structure of the glu119gln m.hhai, c5-cytosine DNA methyltransferase, with unmodified DNA and adohcy
- Reference
- Shieh FK, Reich NO (2007): "AdoMet-dependent Methyl-transfer: Glu(119) Is Essential for DNA C5-Cytosine Methyltransferase M.HhaI." J.Mol.Biol., 373, 1157-1168. doi: 10.1016/j.jmb.2007.08.009.
- Abstract
- The role of Glu119 in S-adenosyl-L-methionine-dependent DNA methyltransferase M.HhaI-catalyzed DNA methylation was studied. Glu119 belongs to the highly conserved Glu/Asn/Val motif found in all DNA C5-cytosine methyltransferases, and its importance for M.HhaI function remains untested. We show that formation of the covalent intermediate between Cys81 and the target cytosine requires Glu119, since conversion to Ala, Asp or Gln lowers the rate of methyl transfer 10(2)-10(6) fold. Further, unlike the wild-type M.HhaI, these mutants are not trapped by the substrate in which the target cytosine is replaced with the mechanism-based inhibitor 5-fluorocytosine. The DNA binding affinity for the Glu119Asp mutant is decreased 10(3)-fold. Thus, the ability of the enzyme to stabilize the extrahelical cytosine is coupled directly to tight DNA binding. The structures of the ternary protein/DNA/AdoHcy complexes for both the Glu119Ala and Glu119Gln mutants (2.70 A and 2.75 A, respectively) show that the flipped base is positioned nearly identically with that observed in the wild-type M.HhaI complex. A single water molecule in the Glu119Ala structure between Ala119 and the extrahelical cytosine N3 is lacking in the Glu119Gln and wild-type M.HhaI structures, and most likely accounts for this mutant's partial activity. Glu119 has essential roles in activating the target cytosine for nucleophilic attack and contributes to tight DNA binding.