Summary information and primary citation
- PDB-id
- 2zi0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-RNA
- Method
- X-ray (2.82 Å)
- Summary
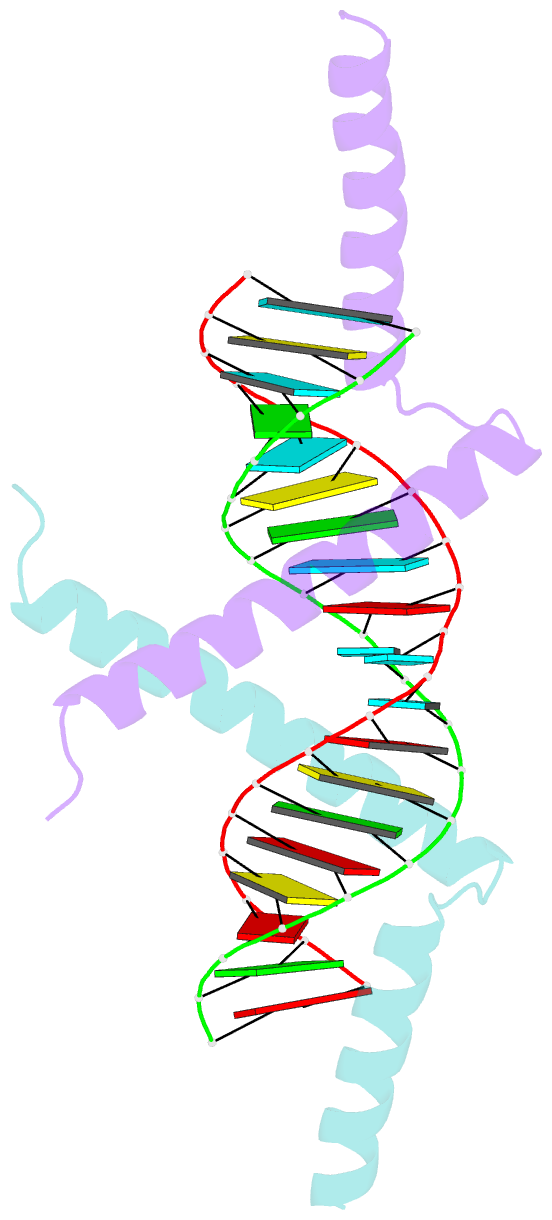
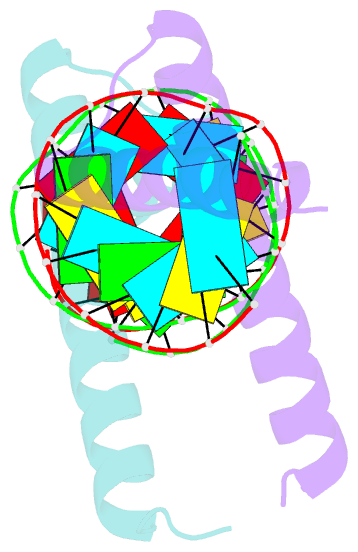
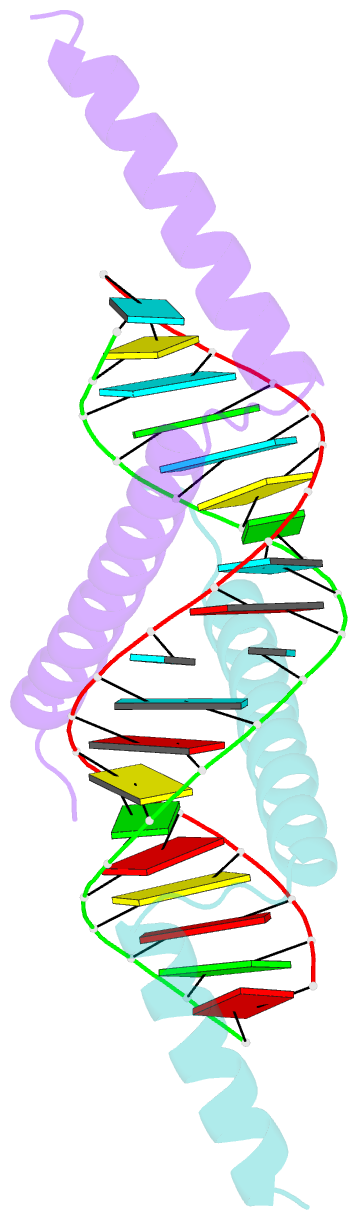
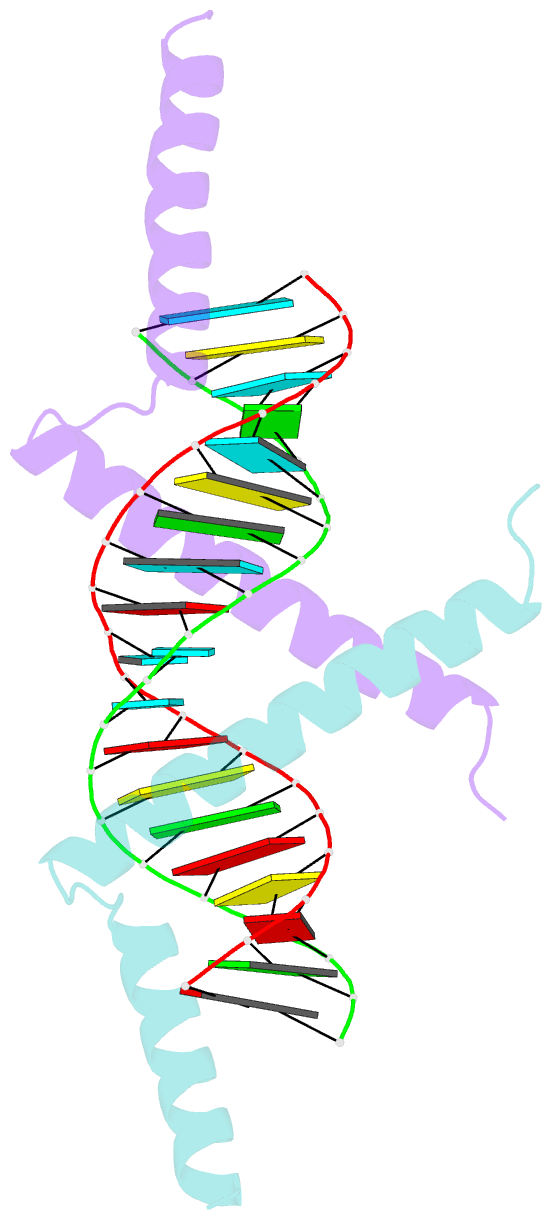
- Crystal structure of tav2b-sirna complex
- Reference
- Chen H-Y, Yang J, Lin C, Yuan YA (2008): "Structural basis for RNA-silencing suppression by Tomato aspermy virus protein 2b." Embo Rep., 9, 754-760. doi: 10.1038/embor.2008.118.
- Abstract
- The 2b proteins encoded by cucumovirus act as post-transcriptional gene silencing suppressors to counter host defence during infection. Here we report the crystal structure of Tomato aspermy virus 2b (TAV2b) protein bound to a 19 bp small interfering RNA (siRNA) duplex. TAV2b adopts an all alpha-helix structure and forms a homodimer to measure siRNA duplex in a length-preference mode. TAV2b has a pair of hook-like structures to recognize simultaneously two alpha-helical turns of A-form RNA duplex by fitting its alpha-helix backbone into two adjacent major grooves of siRNA duplex. The conserved pi-stackings between tryptophan and the 5'-terminal base of siRNA duplex from both ends enhance the recognition. TAV2b further oligomerizes to form a dimer of dimers through the conserved leucine-zipper-like motif at its amino-terminal alpha-helix. Biochemical experiments suggest that TAV2b might interfere with the post-transcriptional gene silencing pathway by directly binding to siRNA duplex.