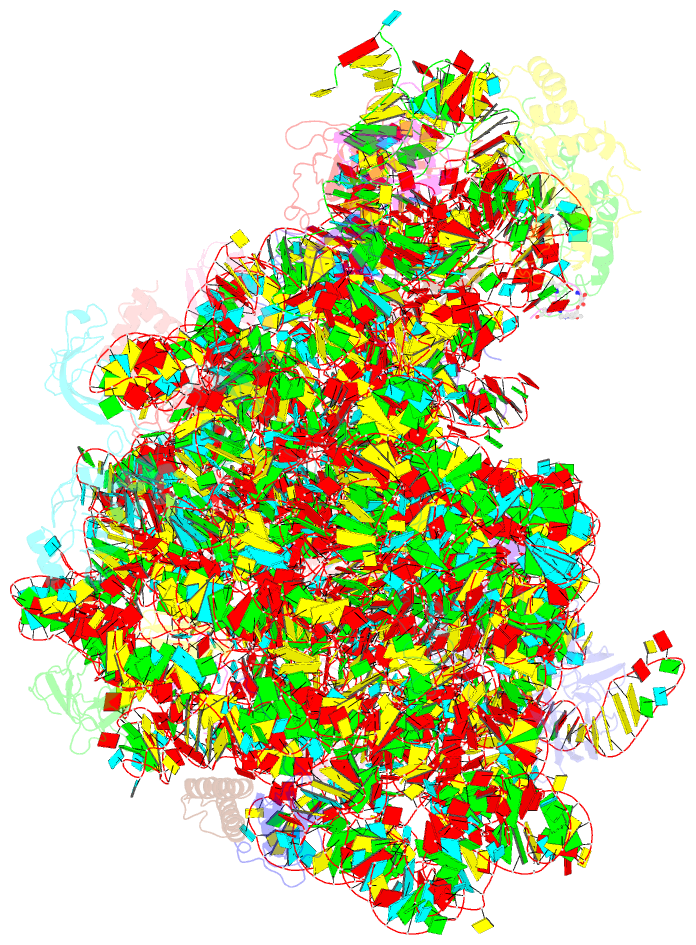
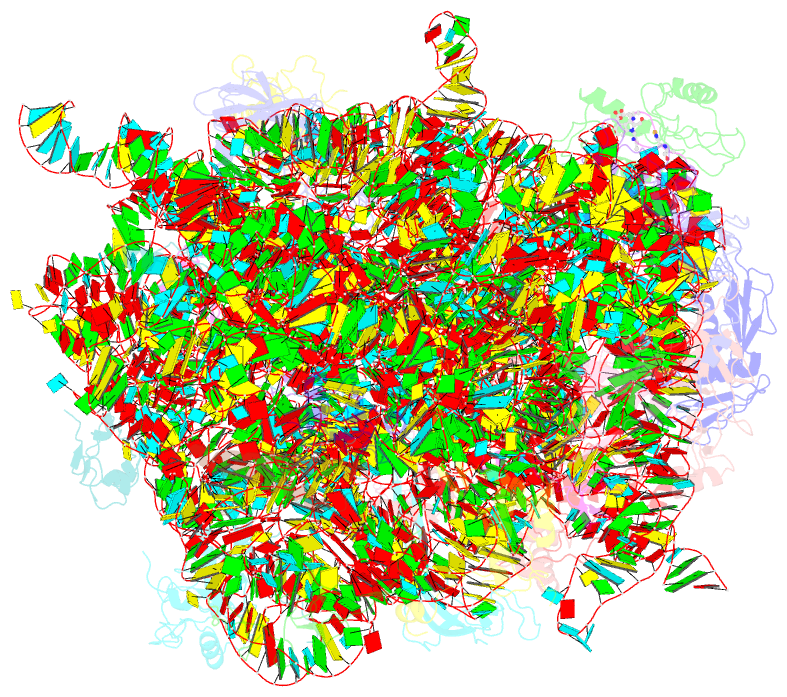
Summary information and primary citation
- PDB-id
- 2zjp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome-antibiotic
- Method
- X-ray (3.7 Å)
- Summary
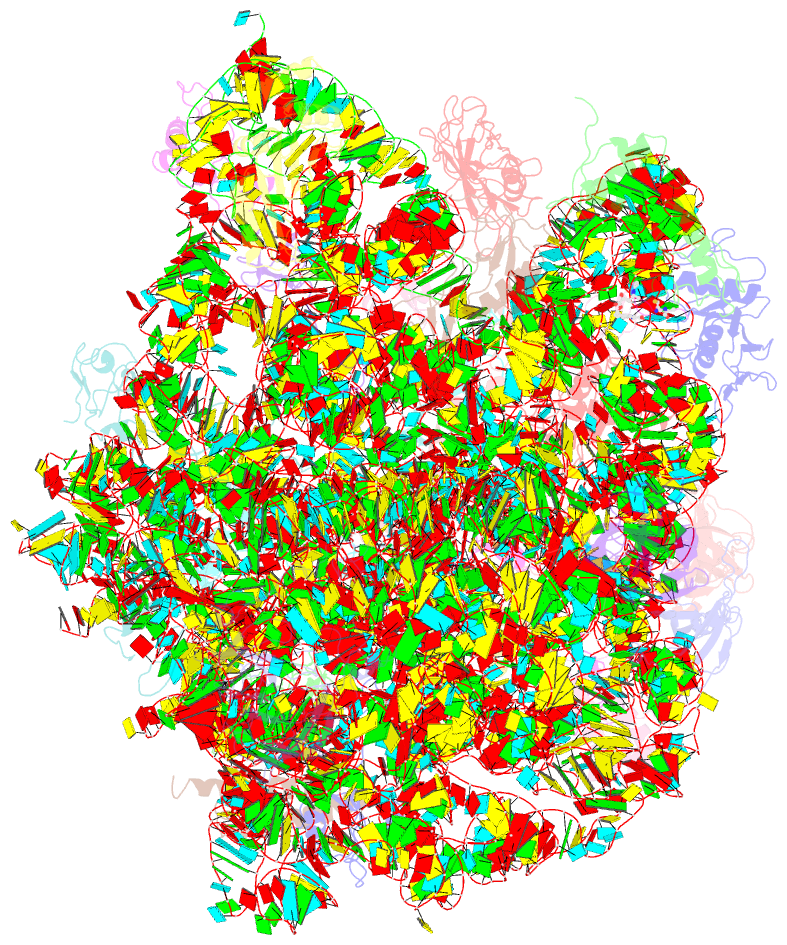
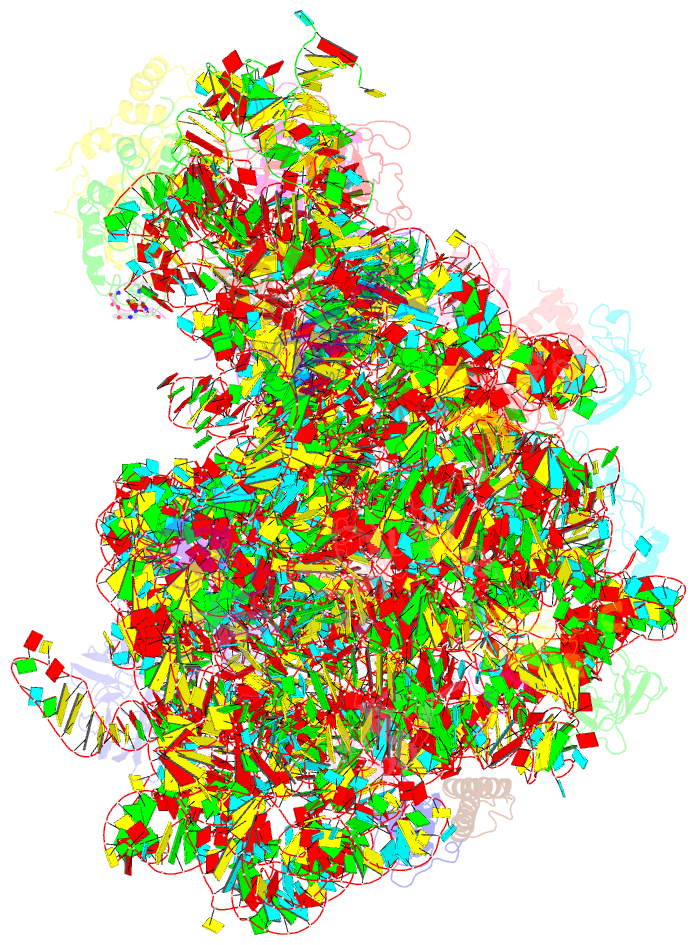
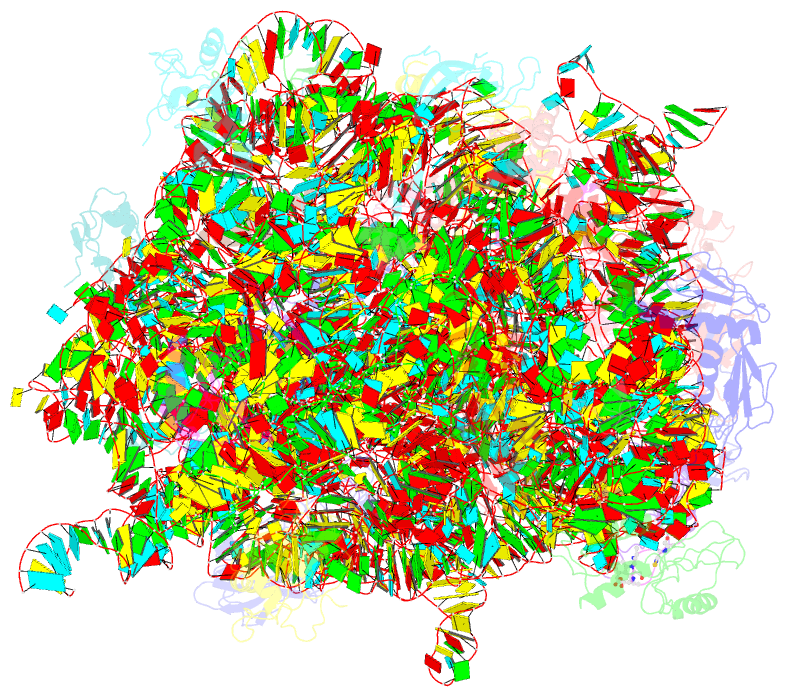
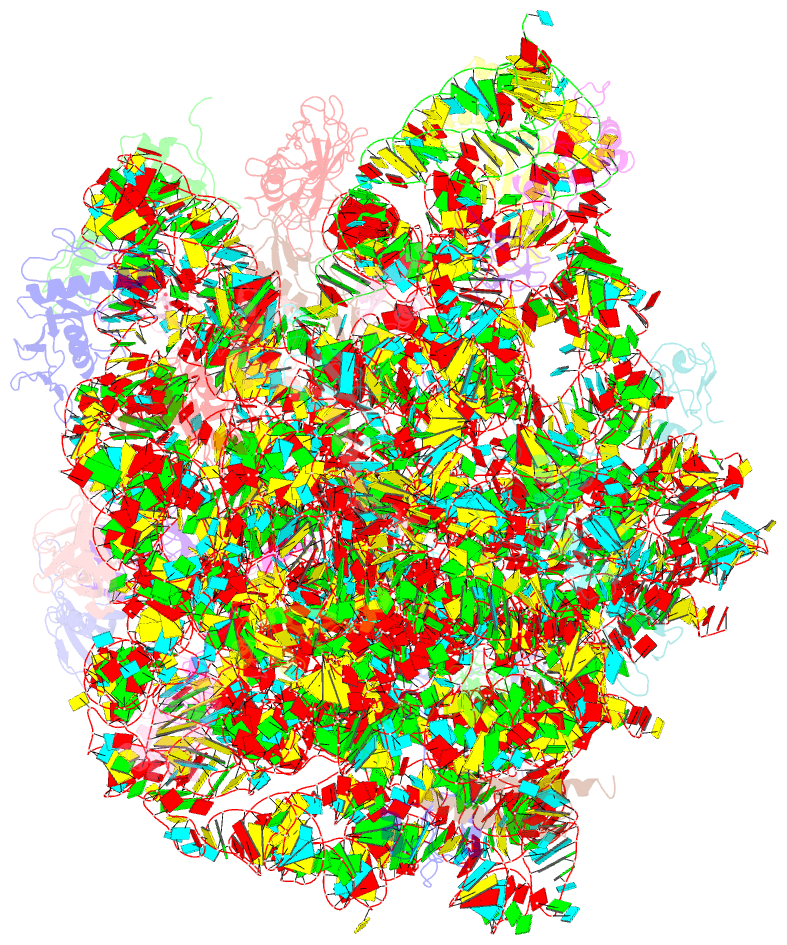
- Thiopeptide antibiotic nosiheptide bound to the large ribosomal subunit of deinococcus radiodurans
- Reference
- Harms JM, Wilson DN, Schluenzen F, Connell SR, Stachelhaus T, Zaborowska Z, Spahn CM, Fucini P (2008): "Translational Regulation Via L11: Molecular Switches on the Ribosome Turned on and Off by Thiostrepton and Micrococcin." Mol.Cell, 30, 26. doi: 10.1016/J.MOLCEL.2008.01.009.
- Abstract
- The thiopeptide class of antibiotics targets the GTPase-associated center (GAC) of the ribosome to inhibit translation factor function. Using X-ray crystallography, we have determined the binding sites of thiostrepton (Thio), nosiheptide (Nosi), and micrococcin (Micro), on the Deinococcus radiodurans large ribosomal subunit. The thiopeptides, by binding within a cleft located between the ribosomal protein L11 and helices 43 and 44 of the 23S rRNA, overlap with the position of domain V of EF-G, thus explaining how this class of drugs perturbs translation factor binding to the ribosome. The presence of Micro leads to additional density for the C-terminal domain (CTD) of L7, adjacent to and interacting with L11. The results suggest that L11 acts as a molecular switch to control L7 binding and plays a pivotal role in positioning one L7-CTD monomer on the G' subdomain of EF-G to regulate EF-G turnover during protein synthesis.