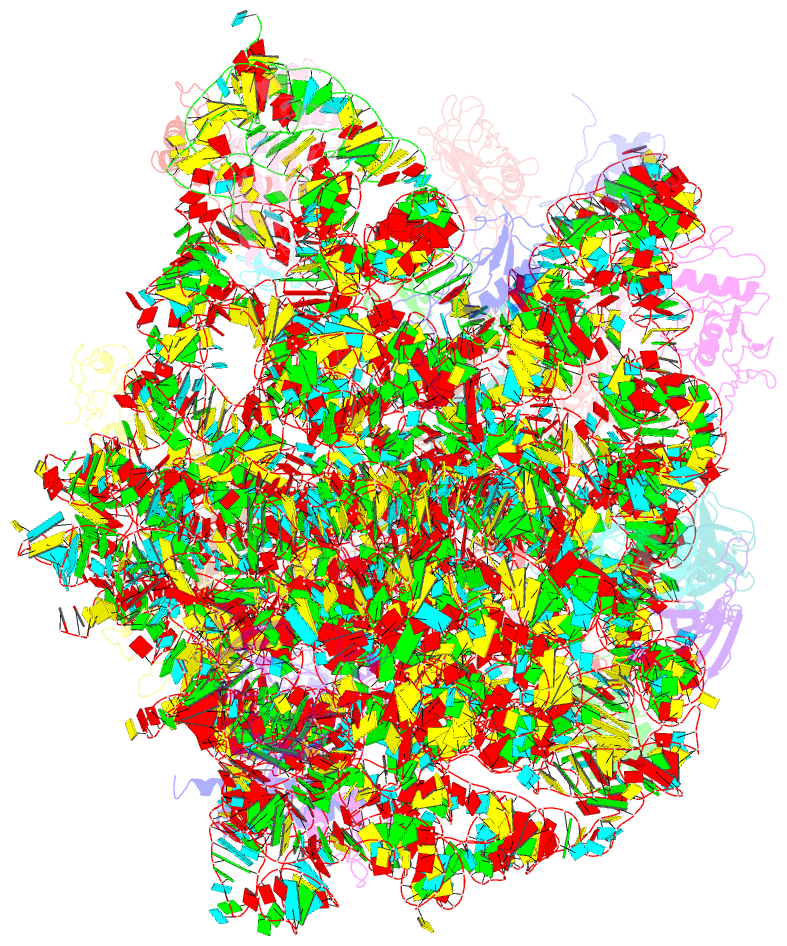
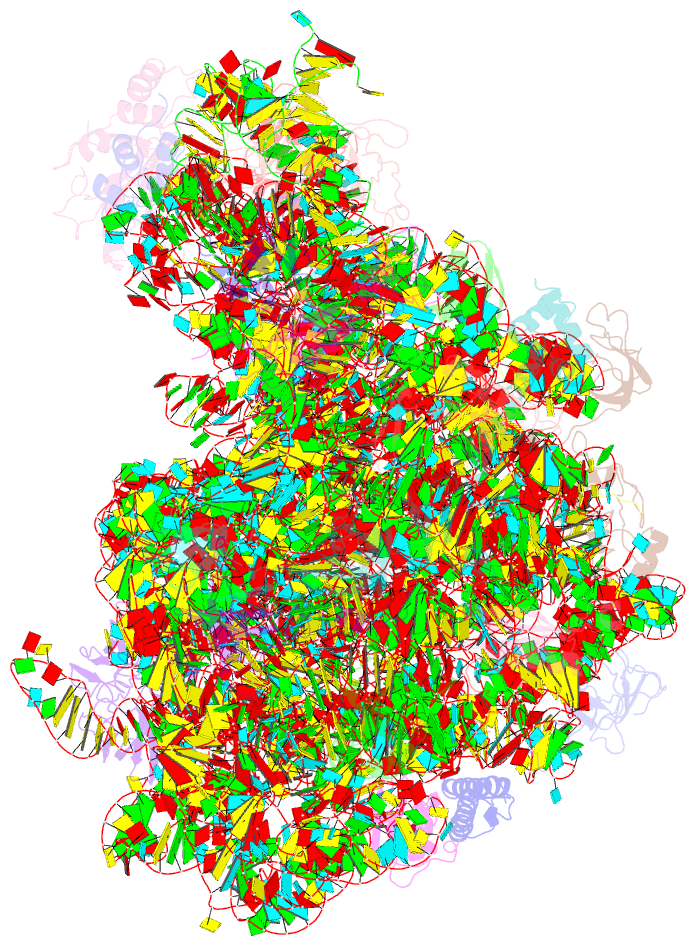
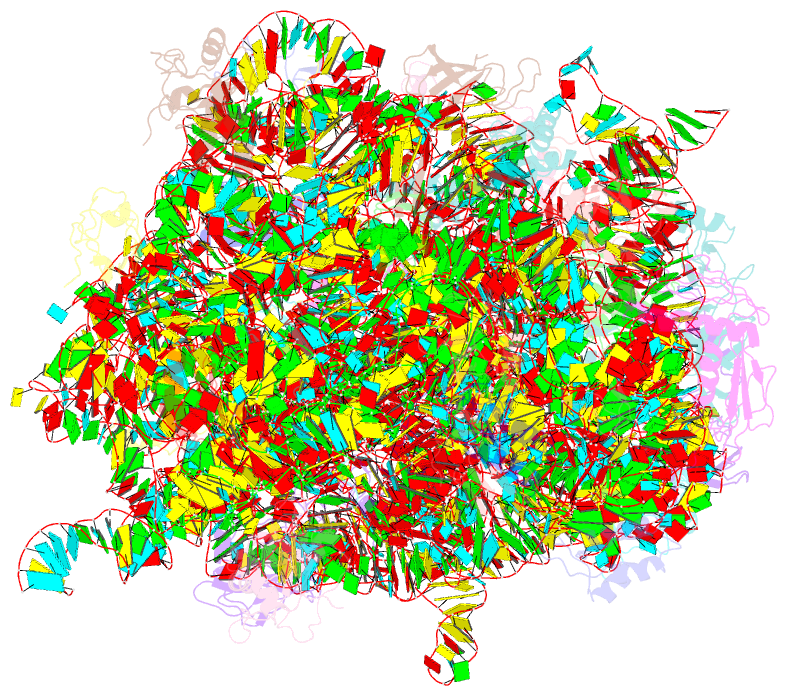
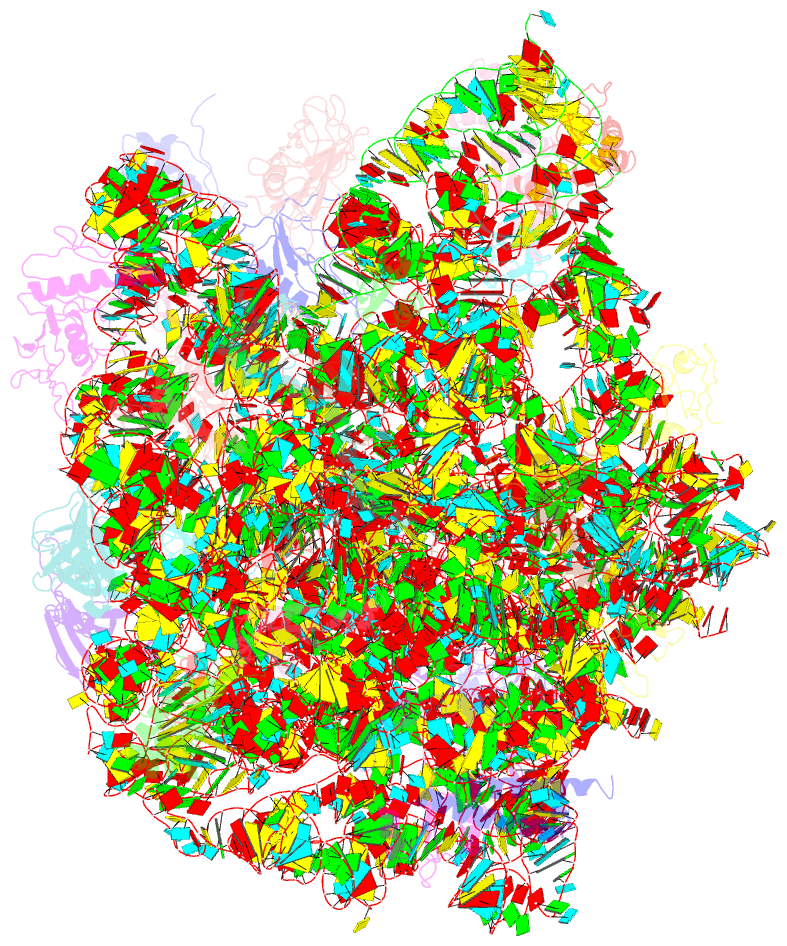
Summary information and primary citation
- PDB-id
- 2zjr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- X-ray (2.91 Å)
- Summary
- Refined native structure of the large ribosomal subunit (50s) from deinococcus radiodurans
- Reference
- Harms JM, Wilson DN, Schluenzen F, Connell SR, Stachelhaus T, Zaborowska Z, Spahn CM, Fucini P (2008): "Translational regulation via L11: molecular switches on the ribosome turned on and off by thiostrepton and micrococcin." Mol.Cell, 30, 26-38. doi: 10.1016/j.molcel.2008.01.009.
- Abstract
- The thiopeptide class of antibiotics targets the GTPase-associated center (GAC) of the ribosome to inhibit translation factor function. Using X-ray crystallography, we have determined the binding sites of thiostrepton (Thio), nosiheptide (Nosi), and micrococcin (Micro), on the Deinococcus radiodurans large ribosomal subunit. The thiopeptides, by binding within a cleft located between the ribosomal protein L11 and helices 43 and 44 of the 23S rRNA, overlap with the position of domain V of EF-G, thus explaining how this class of drugs perturbs translation factor binding to the ribosome. The presence of Micro leads to additional density for the C-terminal domain (CTD) of L7, adjacent to and interacting with L11. The results suggest that L11 acts as a molecular switch to control L7 binding and plays a pivotal role in positioning one L7-CTD monomer on the G' subdomain of EF-G to regulate EF-G turnover during protein synthesis.