Summary information and primary citation
- PDB-id
- 2zko; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (1.7 Å)
- Summary
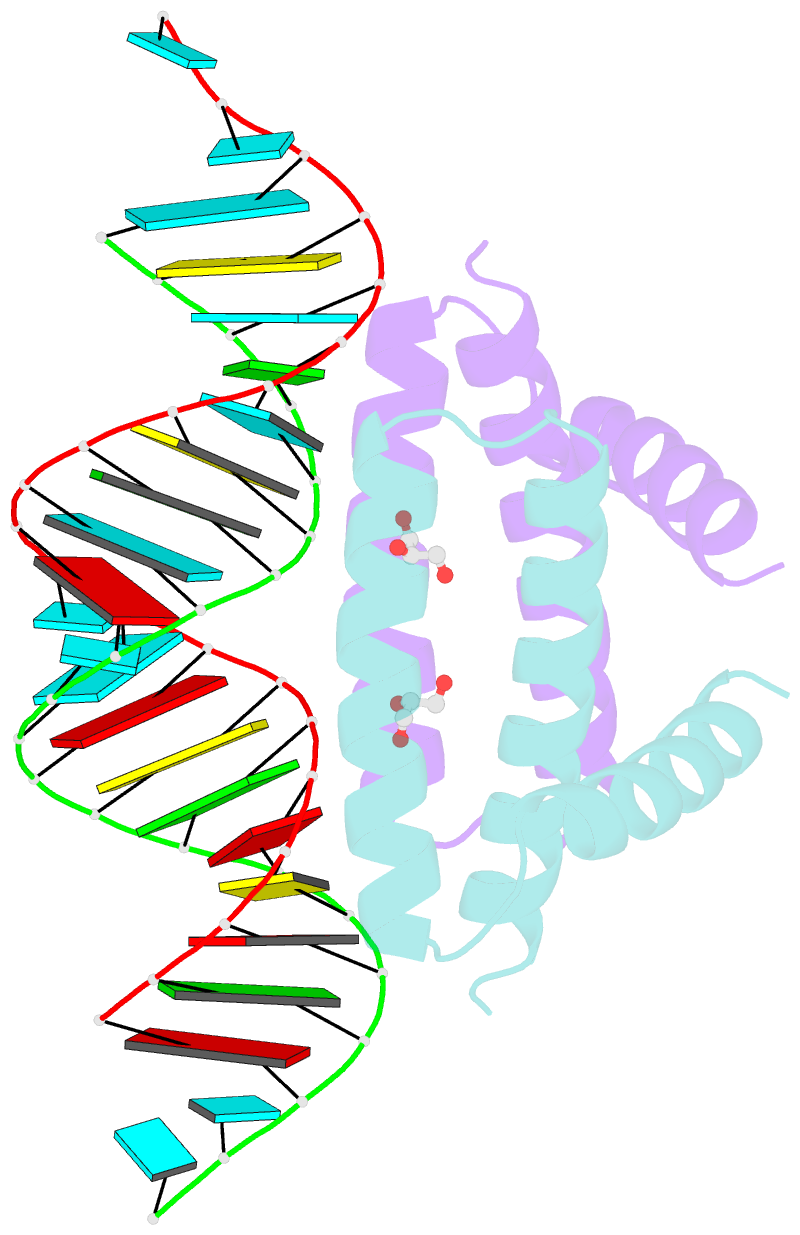
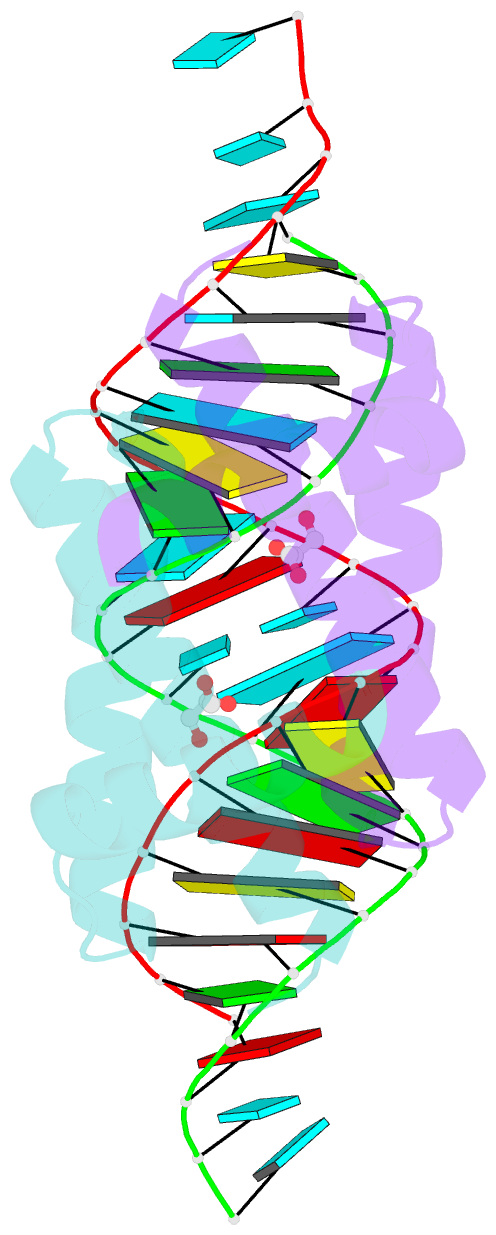
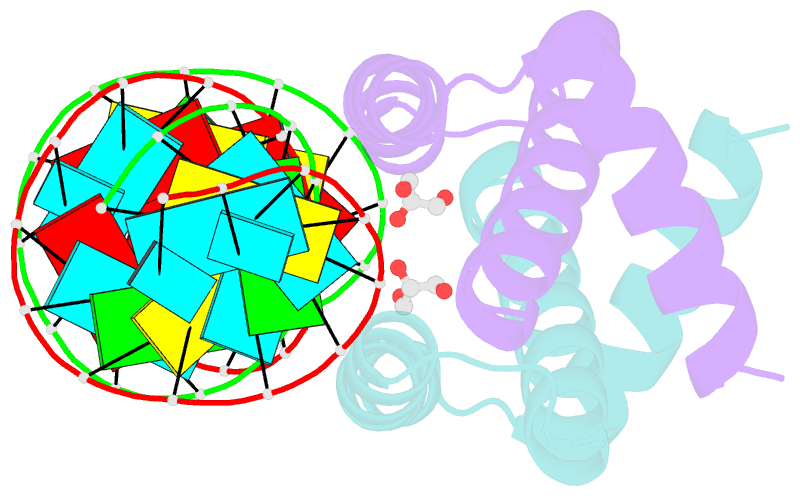
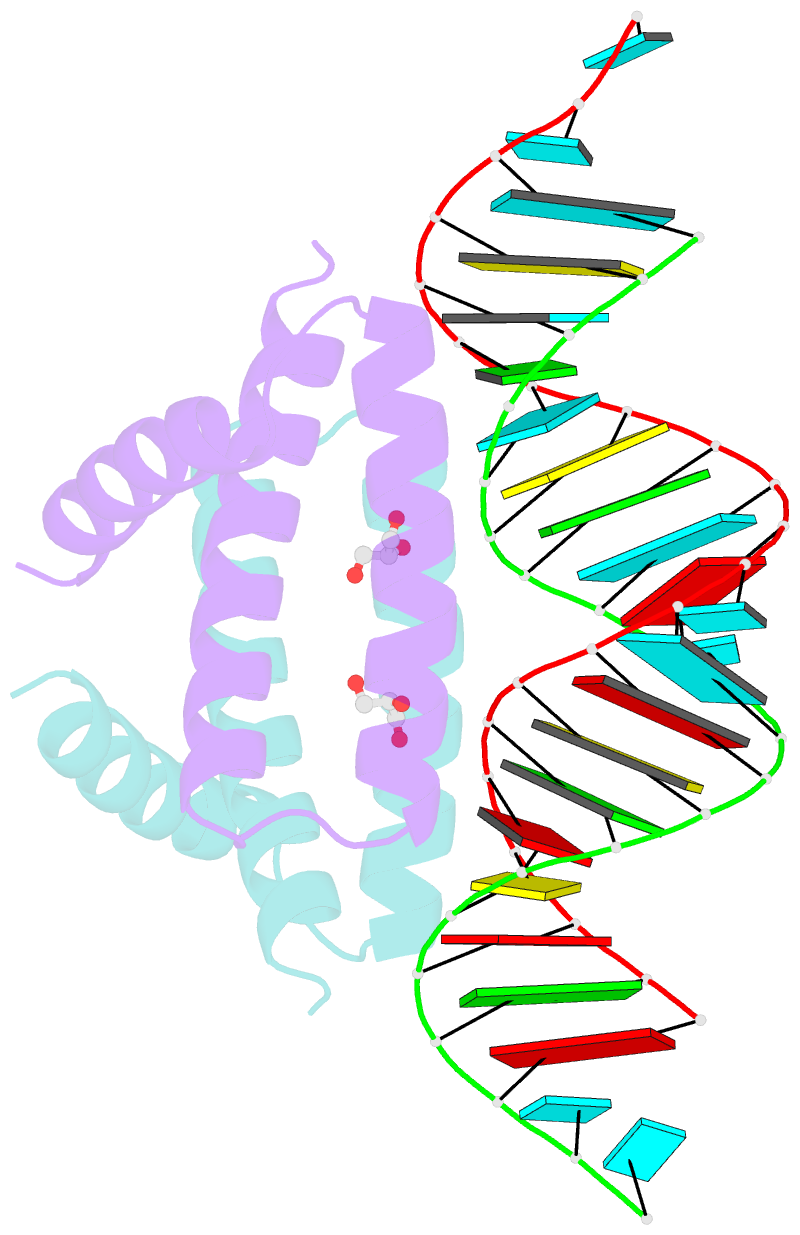
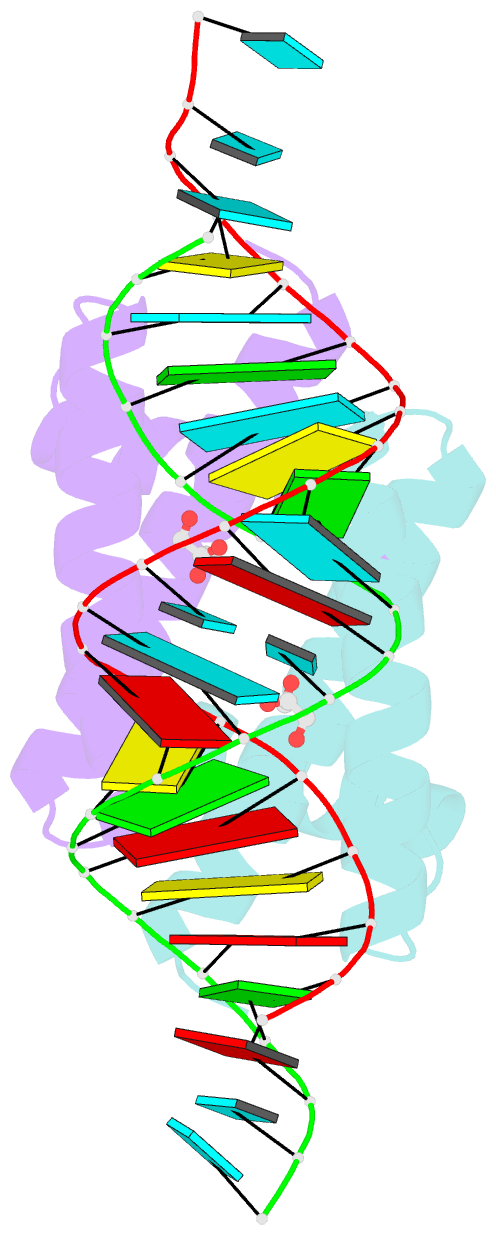
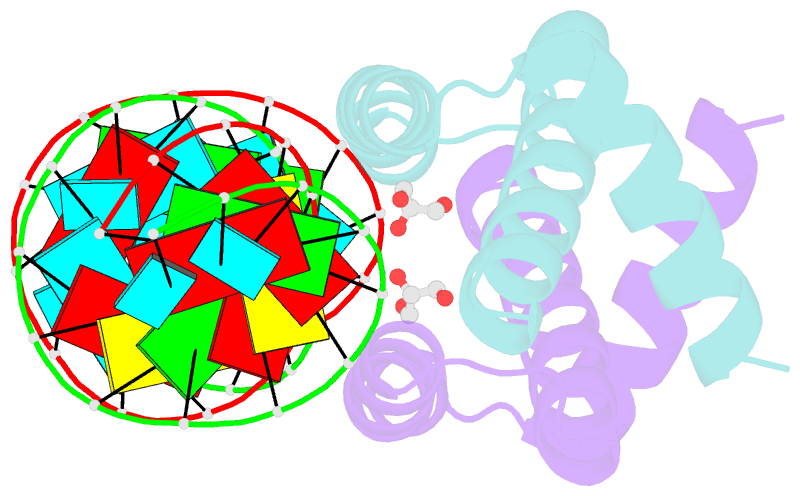
- Structural basis for dsrna recognition by ns1 protein of human influenza virus a
- Reference
- Cheng A, Wong SM, Yuan YA (2009): "Structural basis for dsRNA recognition by NS1 protein of influenza A virus." Cell Res., 19, 187-195. doi: 10.1038/cr.2008.288.
- Abstract
- Influenza A viruses are important human pathogens causing periodic pandemic threats. Nonstructural protein 1 (NS1) protein of influenza A virus (NS1A) shields the virus against host defense. Here, we report the crystal structure of NS1A RNA-binding domain (RBD) bound to a double-stranded RNA (dsRNA) at 1.7A. NS1A RBD forms a homodimer to recognize the major groove of A-form dsRNA in a length-independent mode by its conserved concave surface formed by dimeric anti-parallel alpha-helices. dsRNA is anchored by a pair of invariable arginines (Arg38) from both monomers by extensive hydrogen bonds. In accordance with the structural observation, isothermal titration calorimetry assay shows that the unique Arg38-Arg38 pair and two Arg35-Arg46 pairs are crucial for dsRNA binding, and that Ser42 and Thr49 are also important for dsRNA binding. Agrobacterium co-infiltration assay further supports that the unique Arg38 pair plays important roles in dsRNA binding in vivo.Cell Research (2009) 19:187-195. doi: 10.1038/cr.2008.288; published online 23 September 2008.