Summary information and primary citation
- PDB-id
- 2zzm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-RNA
- Method
- X-ray (2.65 Å)
- Summary
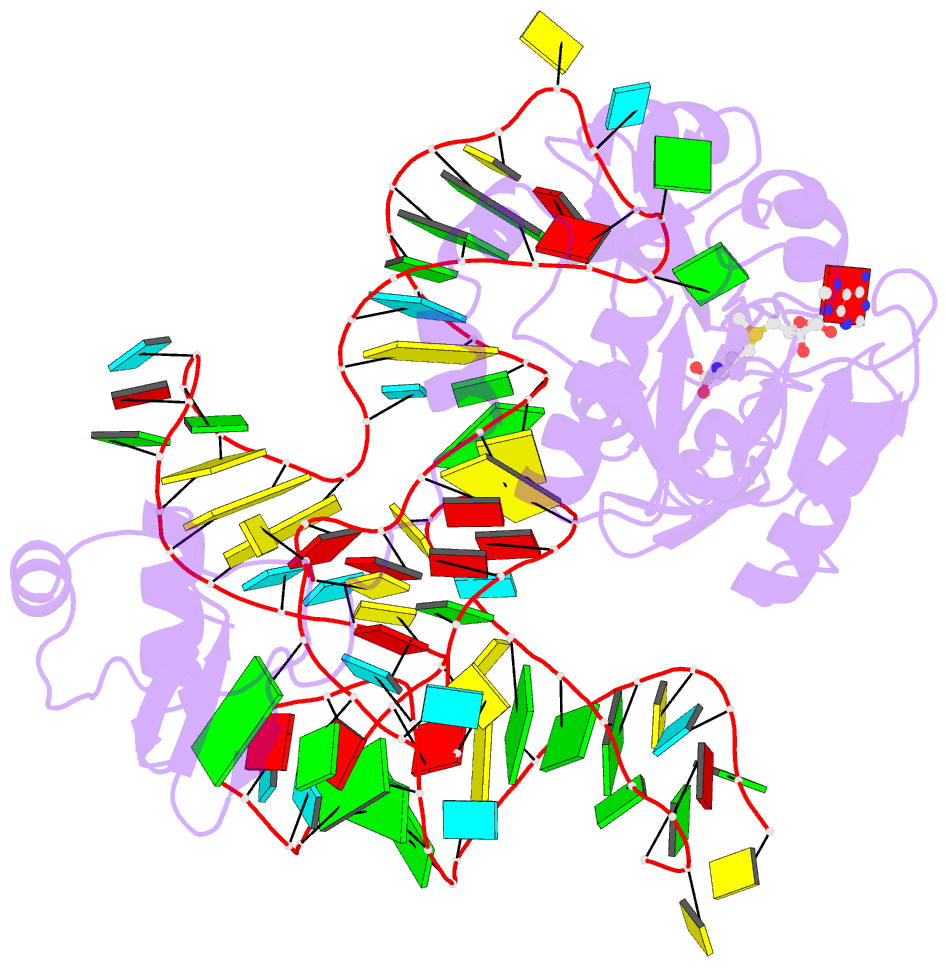
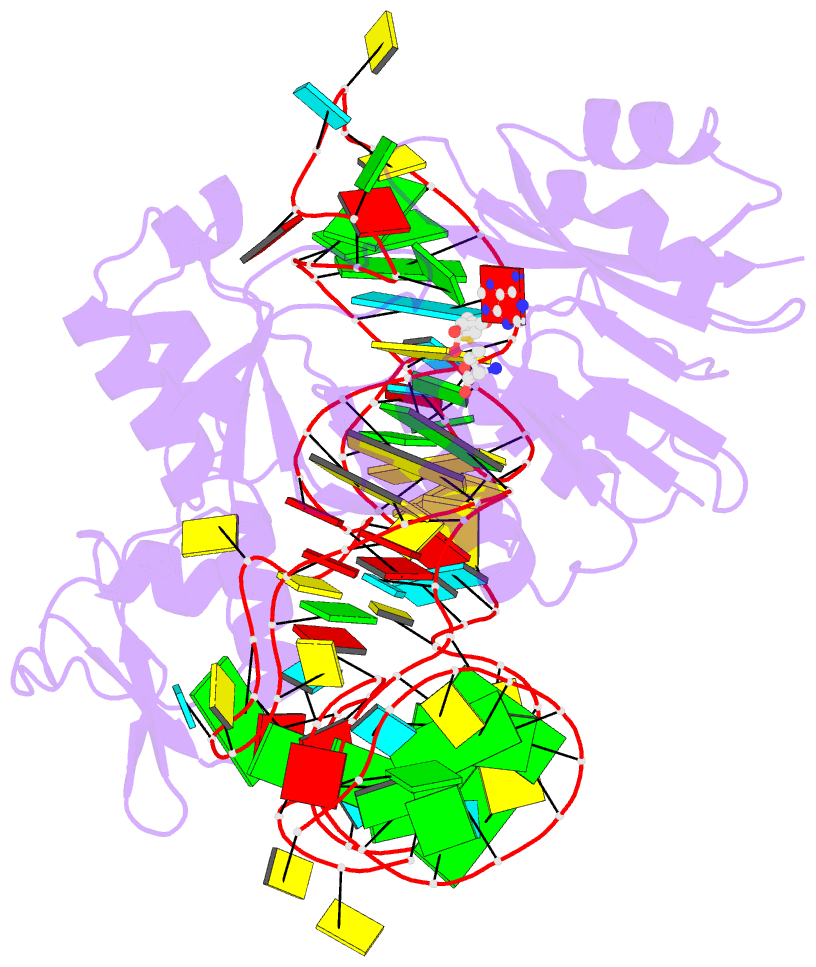
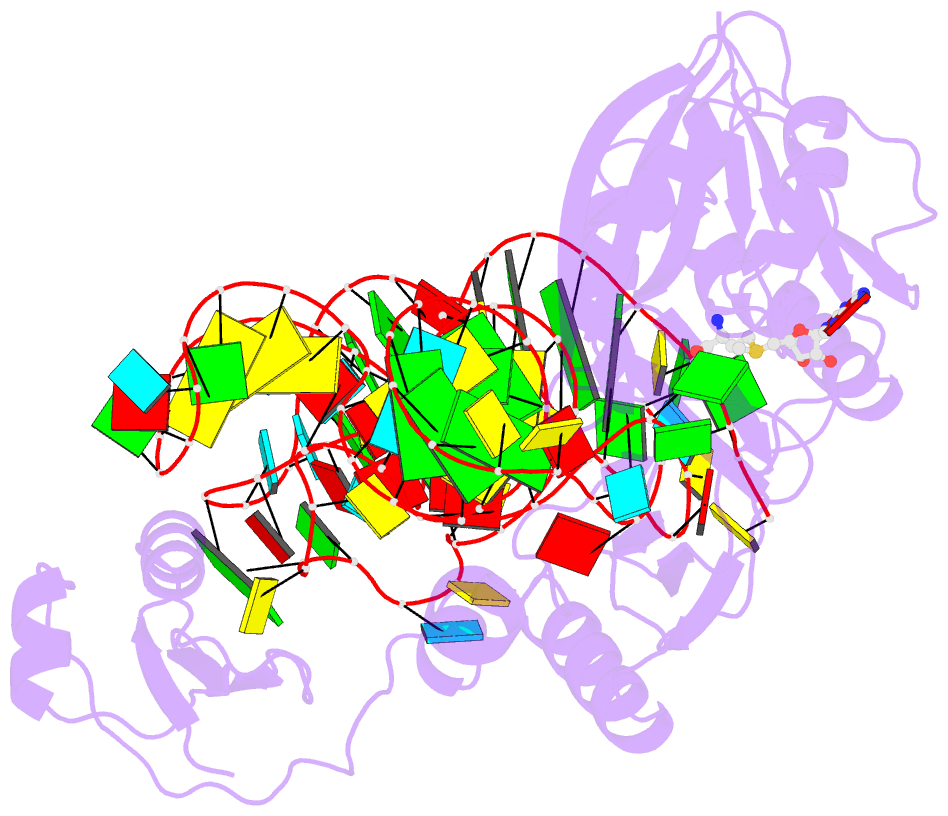
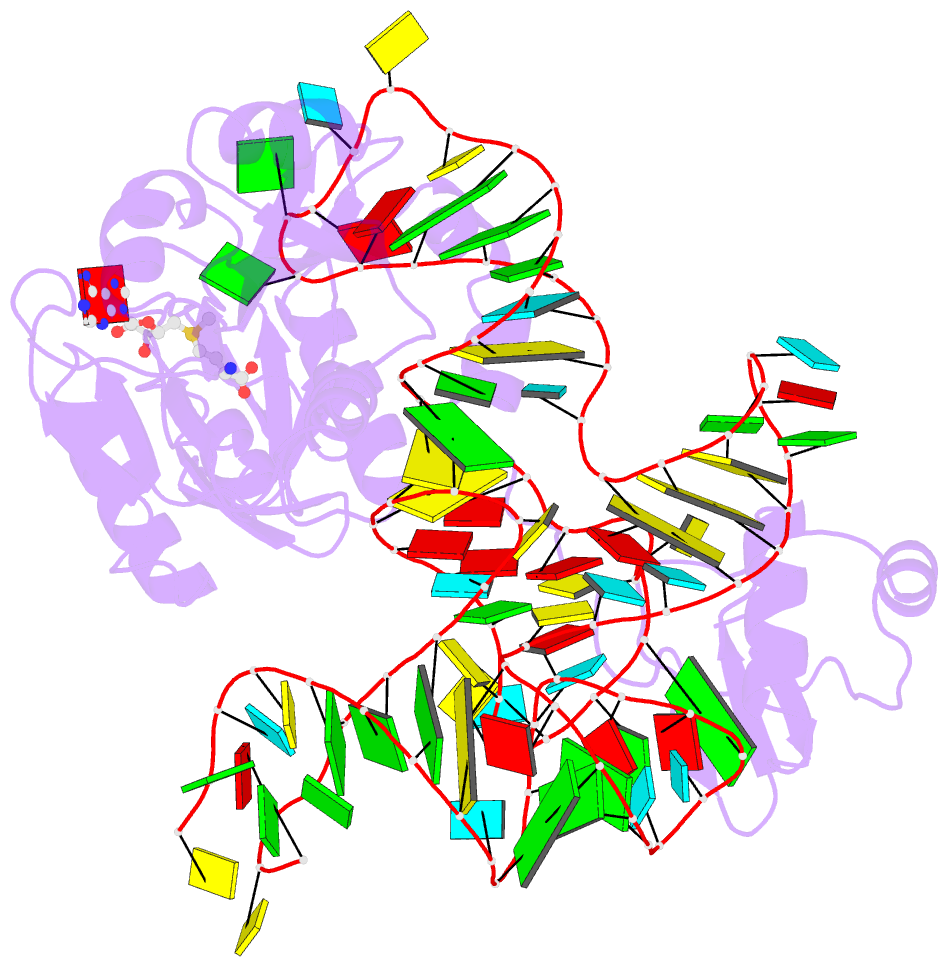
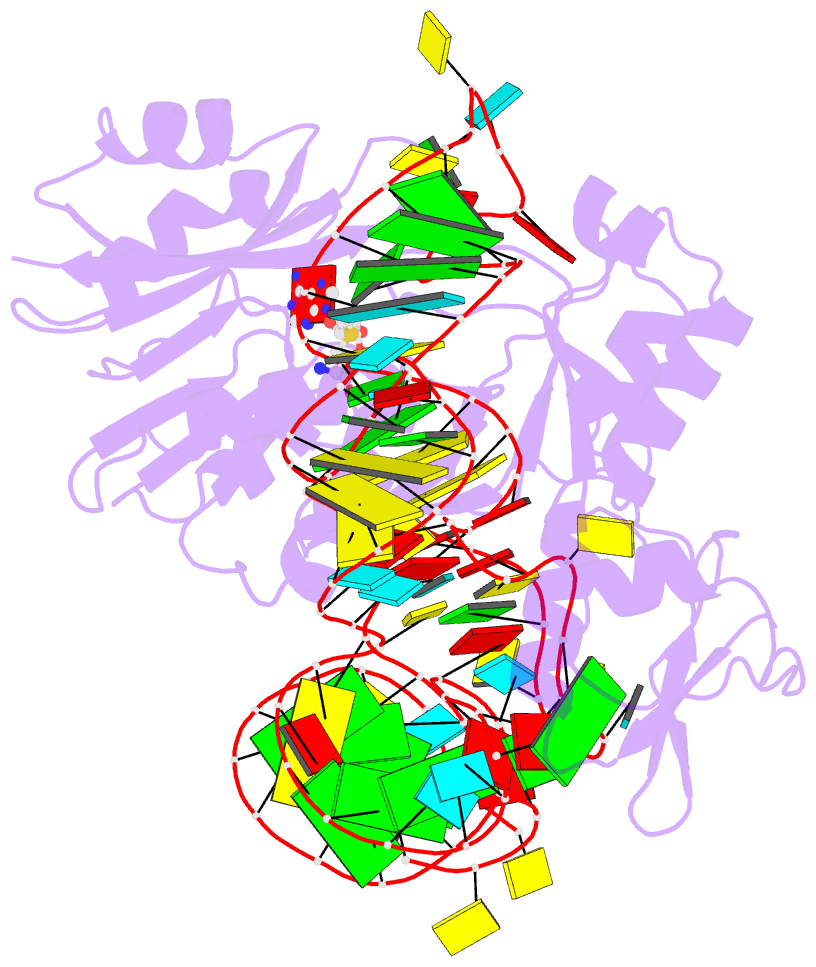
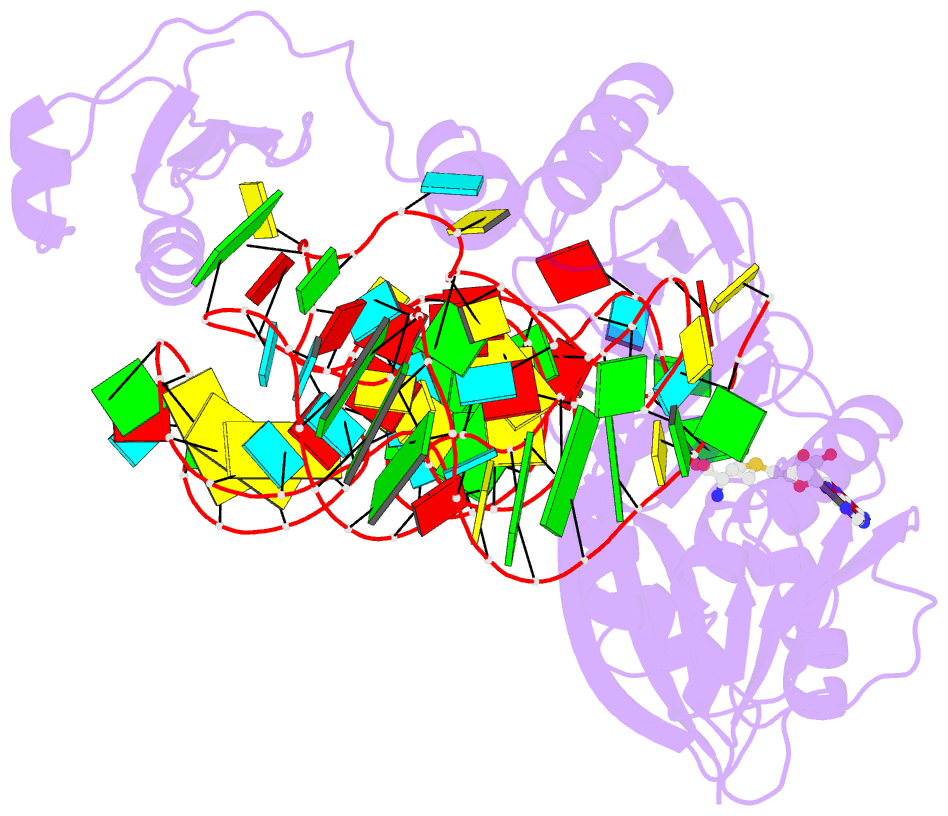
- The complex structure of atrm5 and trnaleu
- Reference
- Goto-Ito S, Ito T, Kuratani M, Bessho Y, Yokoyama S (2009): "Tertiary structure checkpoint at anticodon loop modification in tRNA functional maturation." Nat.Struct.Mol.Biol., 16, 1109-1115. doi: 10.1038/nsmb.1653.
- Abstract
- tRNA precursors undergo a maturation process, involving nucleotide modifications and folding into the L-shaped tertiary structure. The N1-methylguanosine at position 37 (m1G37), 3' adjacent to the anticodon, is essential for translational fidelity and efficiency. In archaea and eukaryotes, Trm5 introduces the m1G37 modification into all tRNAs bearing G37. Here we report the crystal structures of archaeal Trm5 (aTrm5) in complex with tRNA(Leu) or tRNA(Cys). The D2-D3 domains of aTrm5 discover and modify G37, independently of the tRNA sequences. D1 is connected to D2-D3 through a flexible linker and is designed to recognize the shape of the tRNA outer corner, as a hallmark of the completed L shape formation. This interaction by D1 lowers the K(m) value for tRNA, enabling the D2-D3 catalysis. Thus, we propose that aTrm5 provides the tertiary structure checkpoint in tRNA maturation.