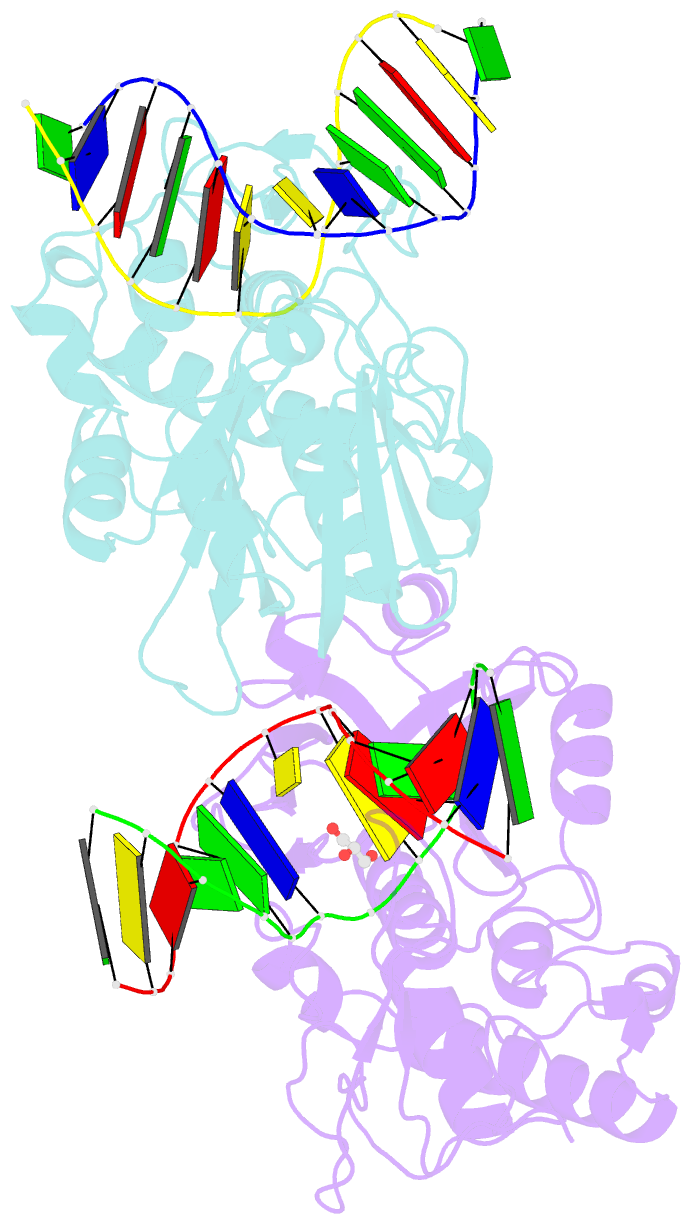
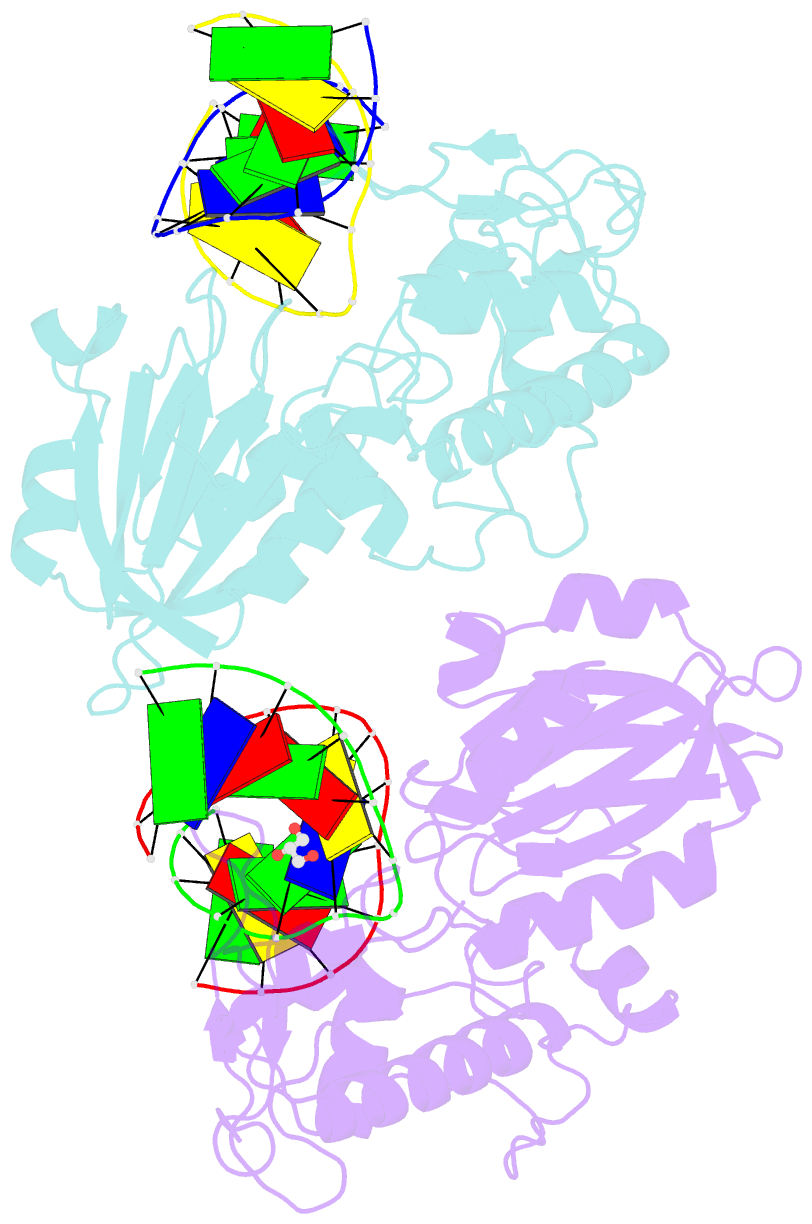
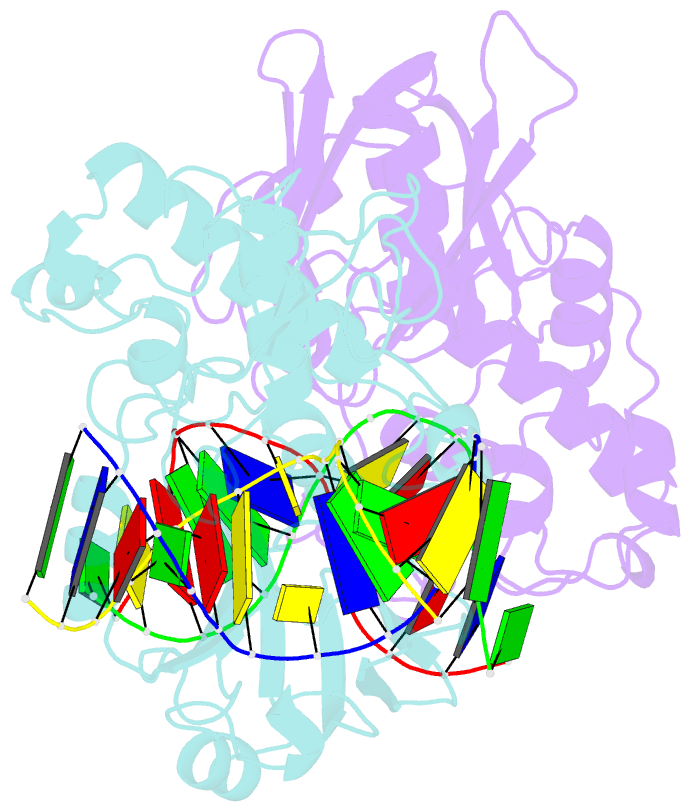
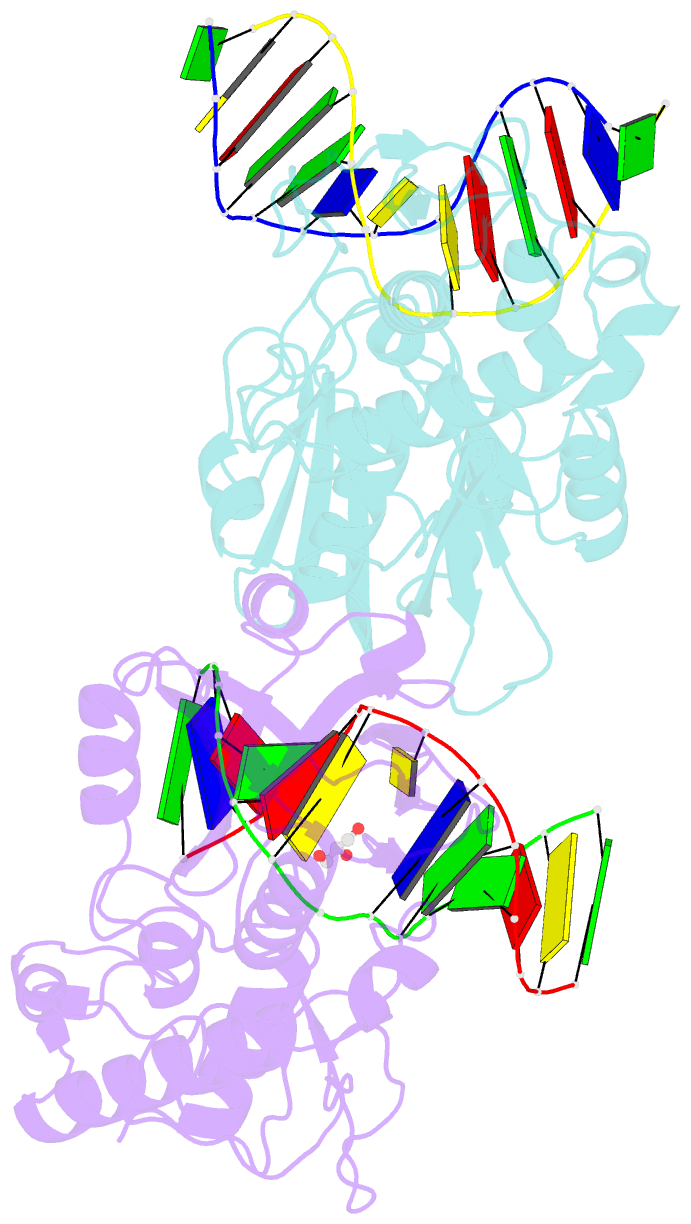
Summary information and primary citation
- PDB-id
- 3a46; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.2 Å)
- Summary
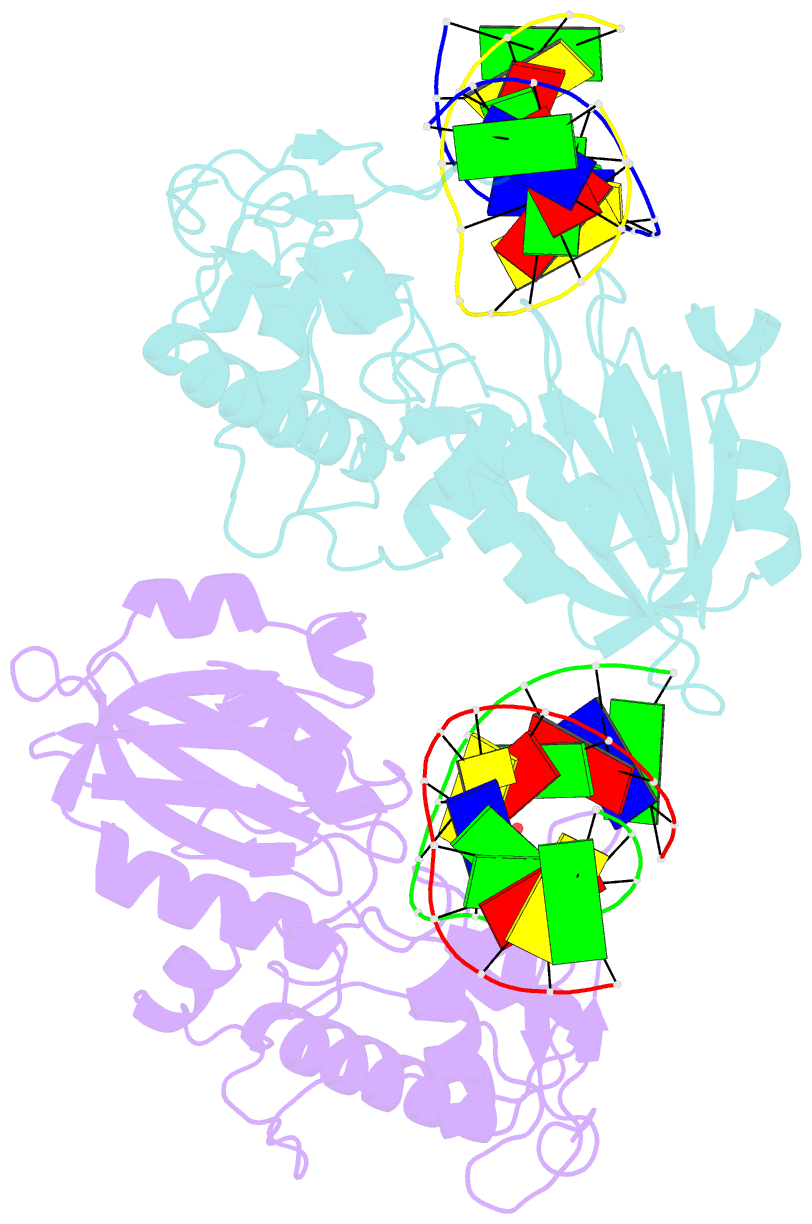
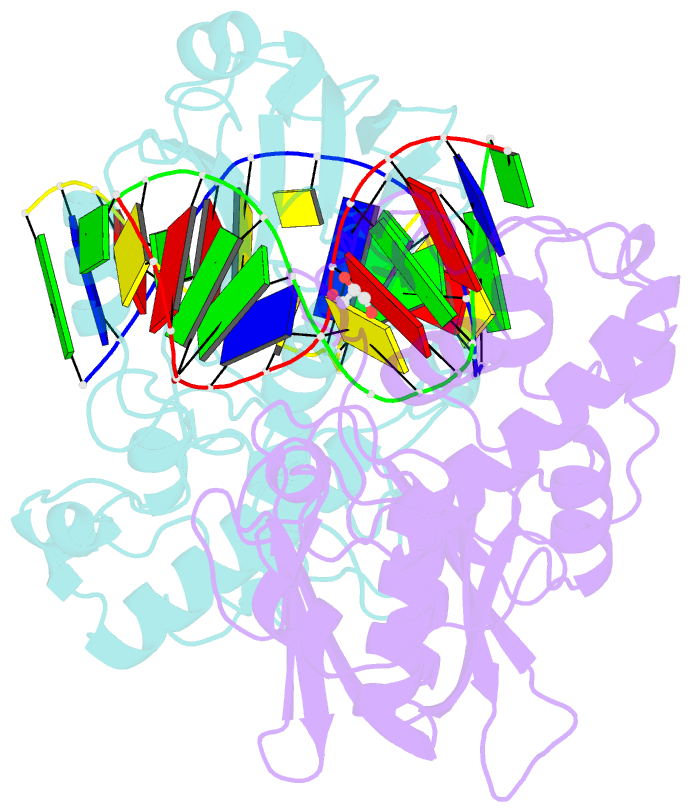
- Crystal structure of mvnei1-thf complex
- Reference
- Imamura K, Wallace SS, Doublie S (2009): "Structural Characterization of a Viral NEIL1 Ortholog Unliganded and Bound to Abasic Site-containing DNA." J.Biol.Chem., 284, 26174-26183. doi: 10.1074/jbc.M109.021907.
- Abstract
- Endonuclease VIII (Nei) is a DNA glycosylase of the base excision repair pathway that recognizes and excises oxidized pyrimidines. We determined the crystal structures of a NEIL1 ortholog from the giant Mimivirus (MvNei1) unliganded and bound to DNA containing tetrahydrofuran (THF), which is the first structure of any Nei with an abasic site analog. The MvNei1 structures exhibit the same overall architecture as other enzymes of the Fpg/Nei family, which consists of two globular domains joined by a linker region. MvNei1 harbors a zincless finger, first described in human NEIL1, rather than the signature zinc finger generally found in the Fpg/Nei family. In contrast to Escherichia coli Nei, where a dramatic conformational change was observed upon binding DNA, the structure of MvNei1 bound to DNA does not reveal any substantial movement compared with the unliganded enzyme. A protein segment encompassing residues 217-245 in MvNei1 corresponds to the "missing loop" in E. coli Nei and the "alphaF-beta10 loop" in E. coli Fpg, which has been reported to be involved in lesion recognition. Interestingly, the corresponding loop in MvNei1 is ordered in both the unliganded and furan-bound structures, unlike other Fpg/Nei enzymes where the loop is generally ordered in the unliganded enzyme or in complexes with a lesion, and disordered otherwise. In the MvNei1.tetrahydrofuran complex a tyrosine located at the tip of the putative lesion recognition loop stacks against the furan ring; the tyrosine is predicted to adopt a different conformation to accommodate a modified base.