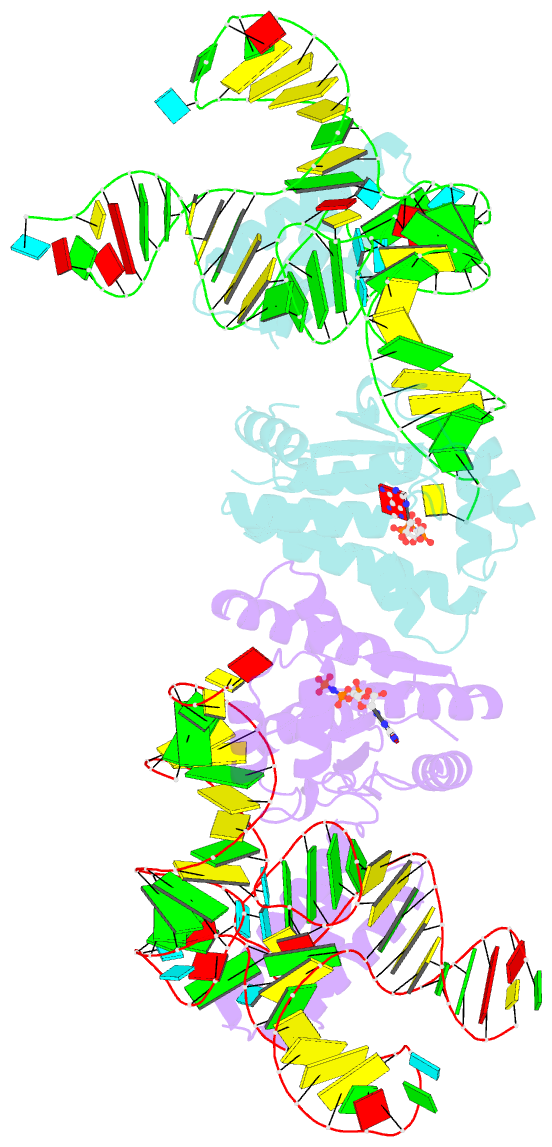
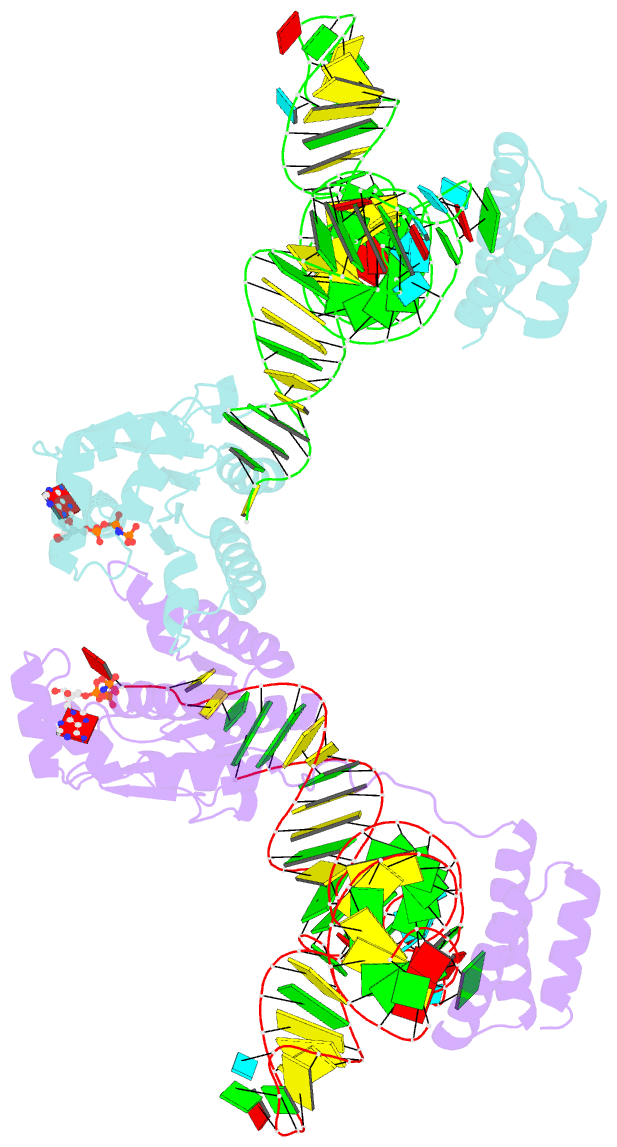
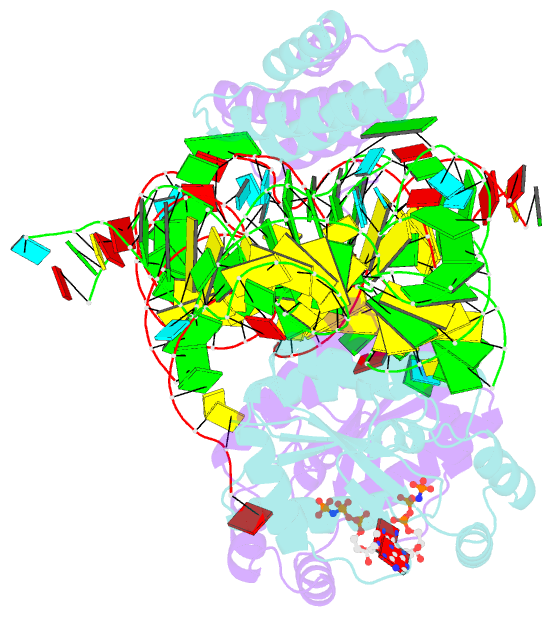
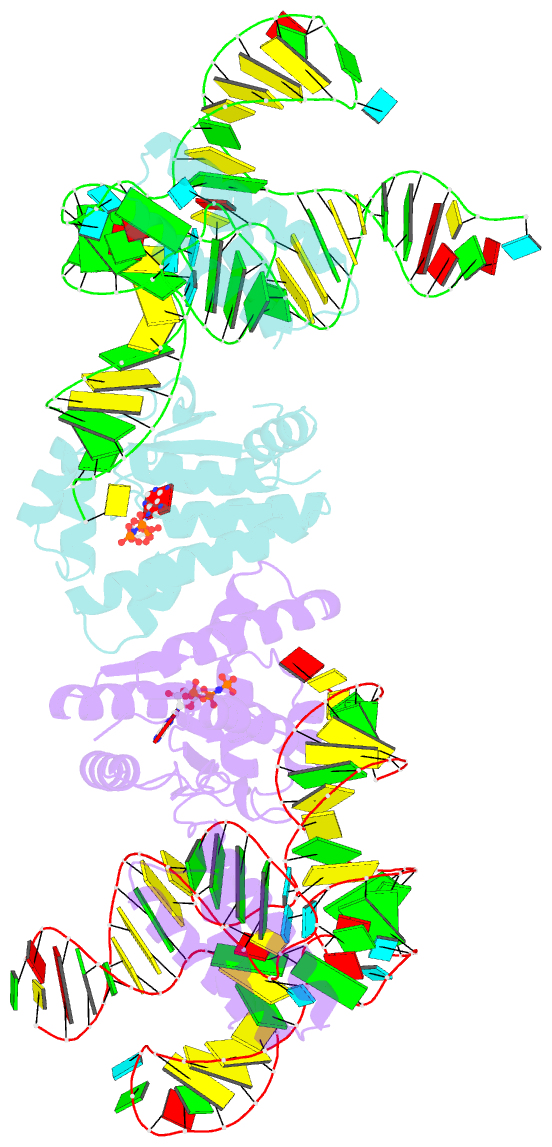
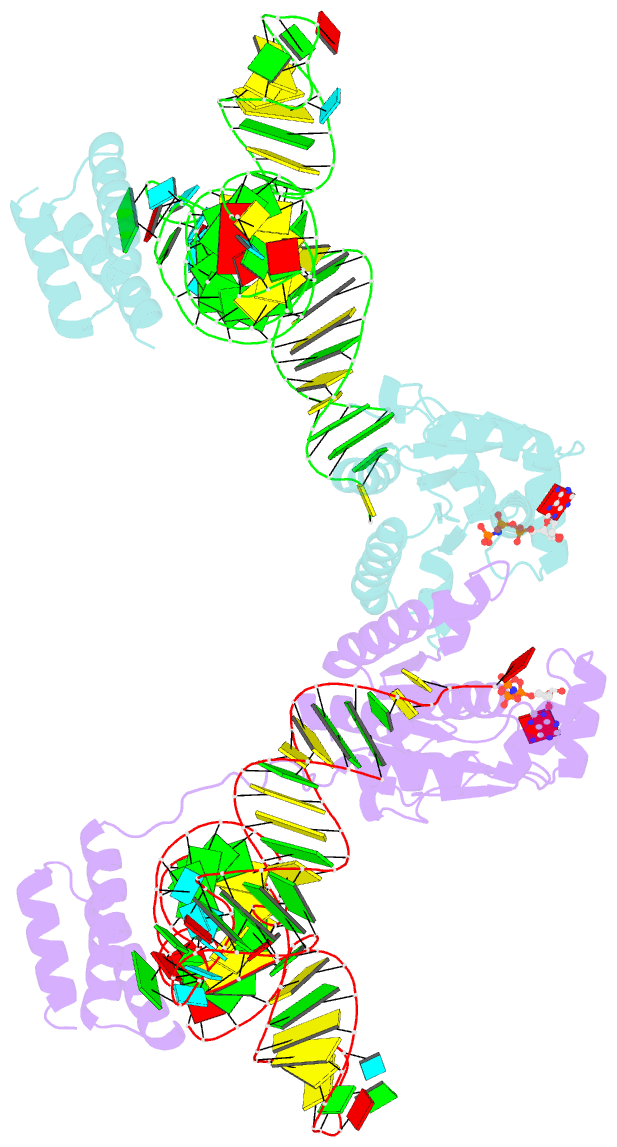
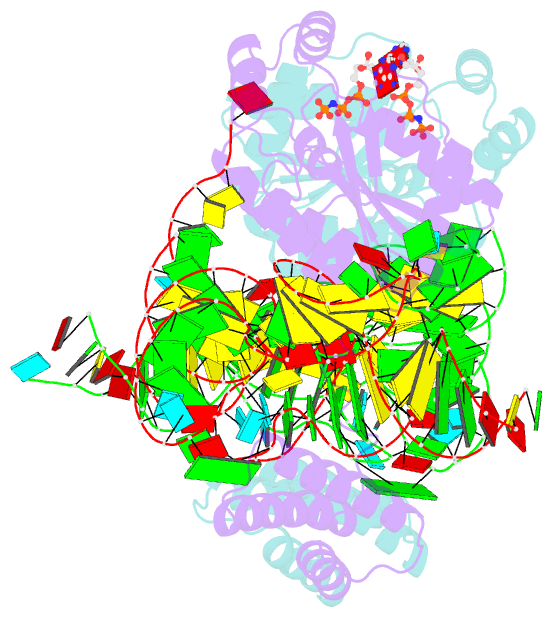
Summary information and primary citation
- PDB-id
- 3add; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-RNA
- Method
- X-ray (2.4 Å)
- Summary
- Crystal structure of o-phosphoseryl-trna kinase complexed with selenocysteine trna and amppnp (crystal type 3)
- Reference
- Chiba S, Itoh Y, Sekine S, Yokoyama S (2010): "Structural Basis for the Major Role of O-Phosphoseryl-tRNA Kinase in the UGA-Specific Encoding of Selenocysteine." Mol.Cell, 39, 410-420. doi: 10.1016/j.molcel.2010.07.018.
- Abstract
- The 21(st) amino acid, selenocysteine (Sec), is assigned to the codon UGA and is biosynthesized on the selenocysteine-specific tRNA (tRNA(Sec)) with the corresponding anticodon. In archaea/eukarya, tRNA(Sec) is ligated with serine by seryl-tRNA synthetase (SerRS), the seryl moiety is phosphorylated by O-phosphoseryl-tRNA kinase (PSTK), and the phosphate group is replaced with selenol by Sep-tRNA:Sec-tRNA synthase. PSTK selectively phosphorylates seryl-tRNA(Sec), while SerRS serylates both tRNA(Ser) and tRNA(Sec). In this study, we determined the crystal structures of the archaeal tRNA(Sec).PSTK complex. PSTK consists of two independent linker-connected domains, the N-terminal catalytic domain (NTD) and the C-terminal domain (CTD). The D-arm.CTD binding occurs independently of and much more strongly than the acceptor-arm.NTD binding. PSTK thereby distinguishes the characteristic D arm with the maximal stem and the minimal loop of tRNA(Sec) from the canonical D arm of tRNA(Ser), without interacting with the anticodon. This mechanism is essential for the UGA-specific encoding of selenocysteine.