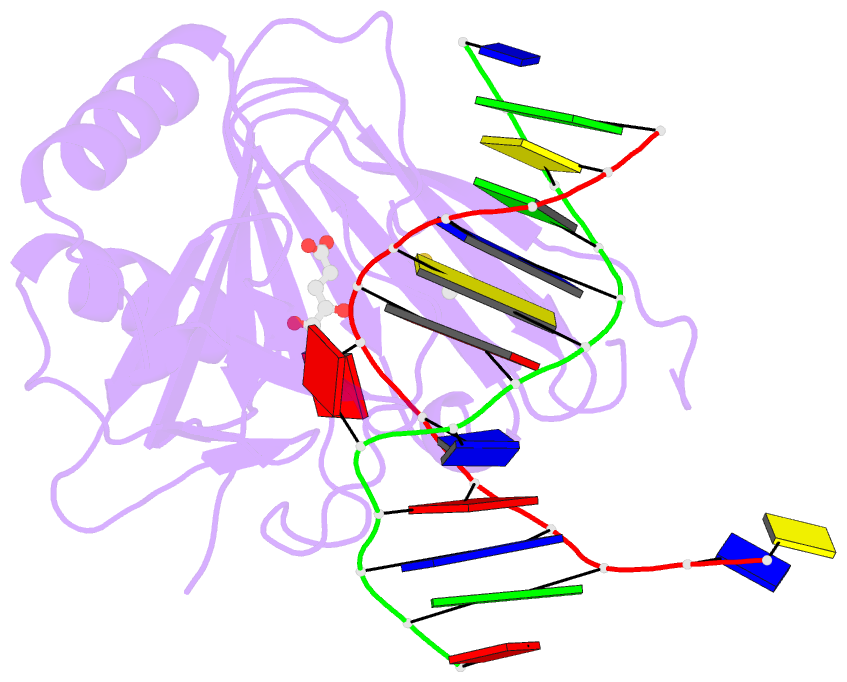
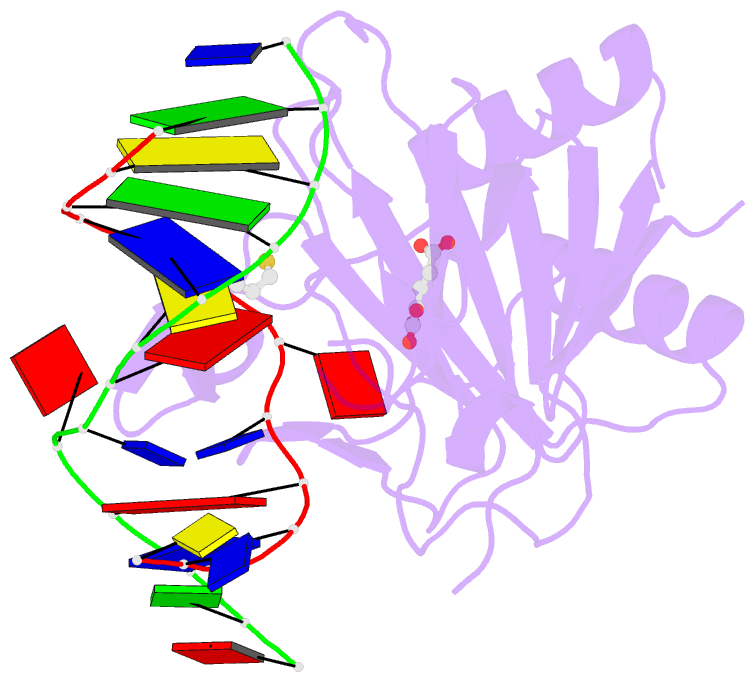
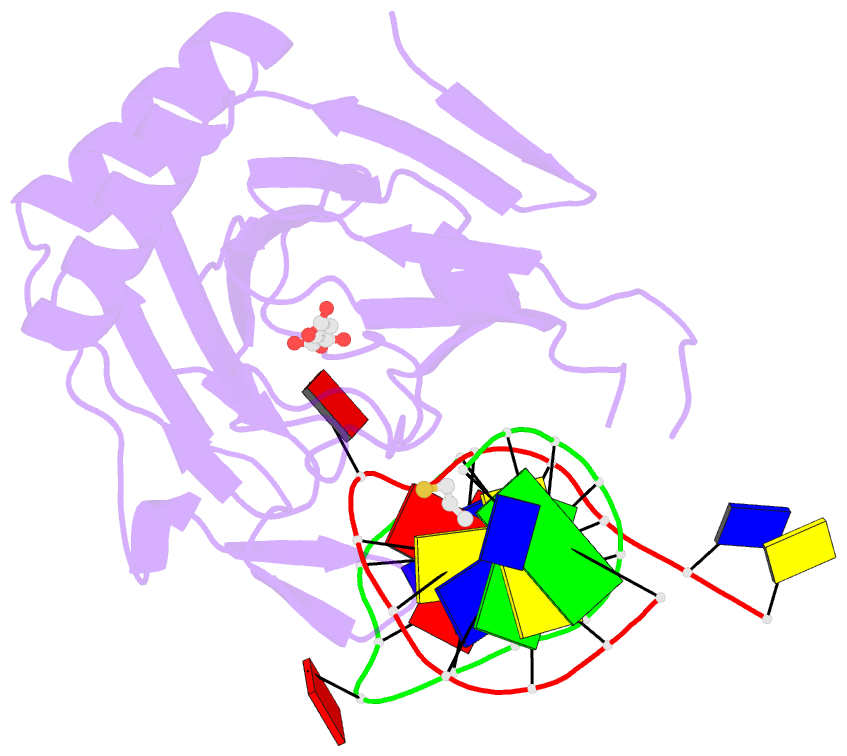
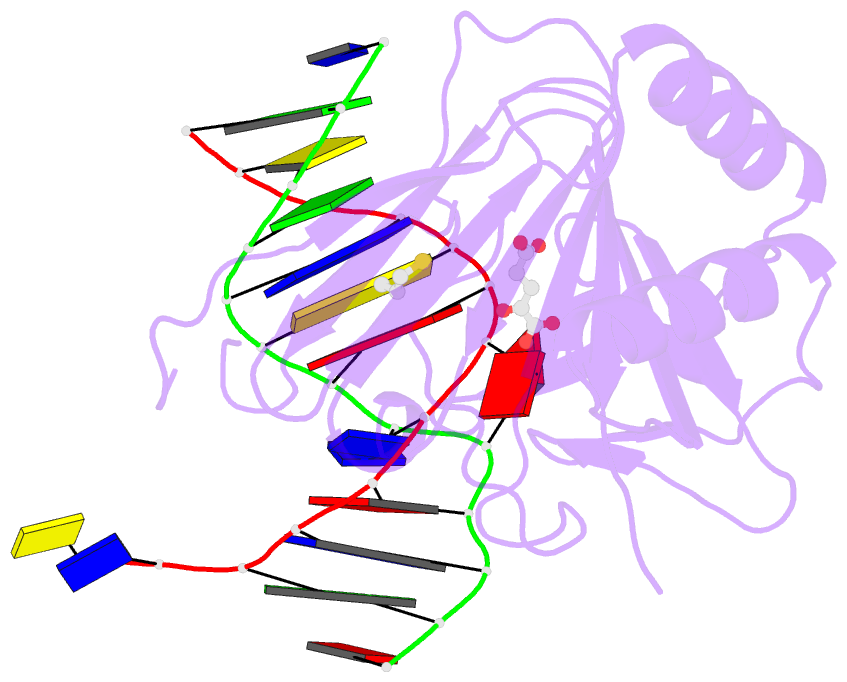
Summary information and primary citation
- PDB-id
- 3buc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- oxidoreductase-DNA
- Method
- X-ray (2.59 Å)
- Summary
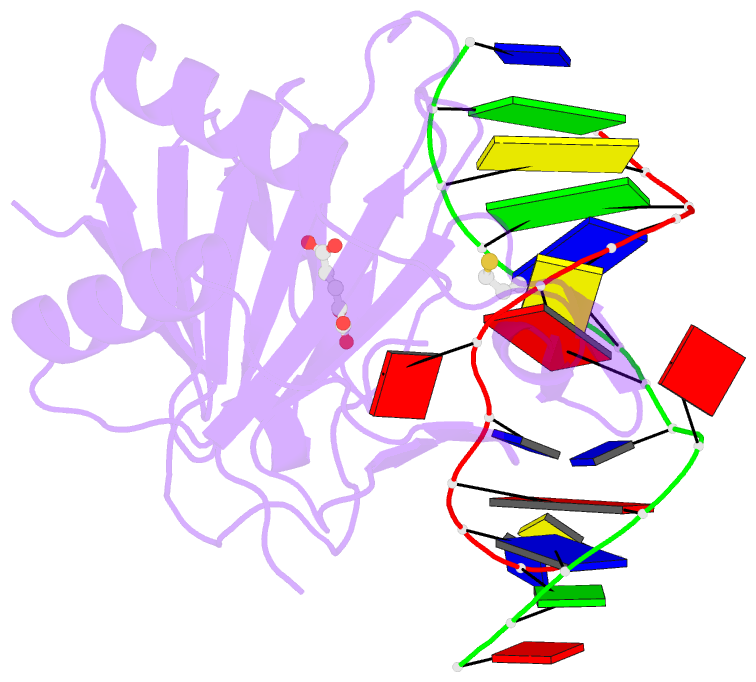
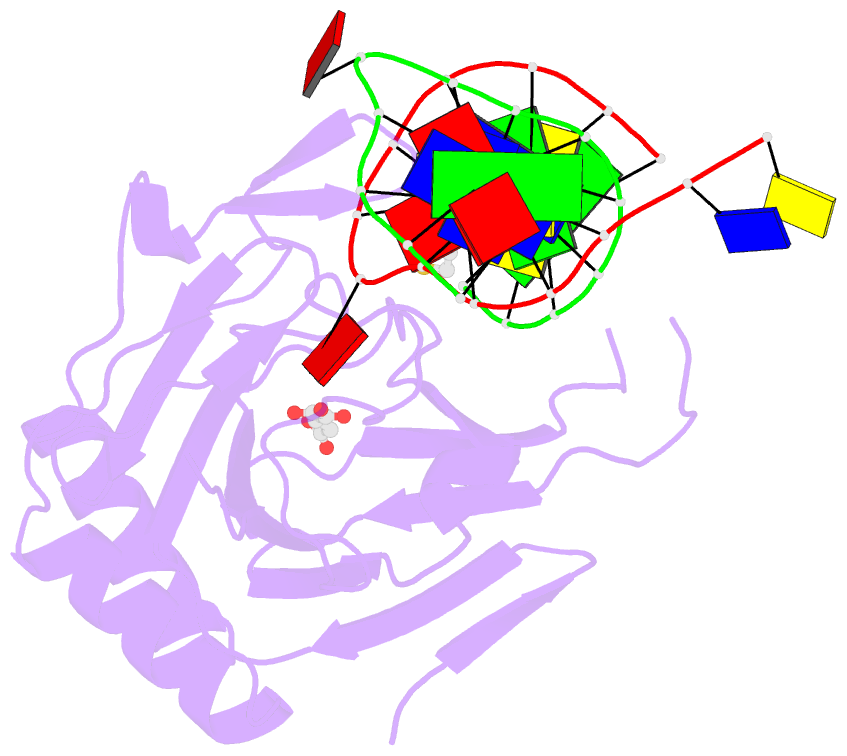
- X-ray structure of human abh2 bound to dsDNA with mn(ii) and 2kg
- Reference
- Yang CG, Yi C, Duguid EM, Sullivan CT, Jian X, Rice PA, He C (2008): "Crystal structures of DNA/RNA repair enzymes AlkB and ABH2 bound to dsDNA." Nature, 452, 961-965. doi: 10.1038/nature06889.
- Abstract
- Escherichia coli AlkB and its human homologues ABH2 and ABH3 repair DNA/RNA base lesions by using a direct oxidative dealkylation mechanism. ABH2 has the primary role of guarding mammalian genomes against 1-meA damage by repairing this lesion in double-stranded DNA (dsDNA), whereas AlkB and ABH3 preferentially repair single-stranded DNA (ssDNA) lesions and can repair damaged bases in RNA. Here we show the first crystal structures of AlkB-dsDNA and ABH2-dsDNA complexes, stabilized by a chemical cross-linking strategy. This study reveals that AlkB uses an unprecedented base-flipping mechanism to access the damaged base: it squeezes together the two bases flanking the flipped-out one to maintain the base stack, explaining the preference of AlkB for repairing ssDNA lesions over dsDNA ones. In addition, the first crystal structure of ABH2, presented here, provides a structural basis for designing inhibitors of this human DNA repair protein.