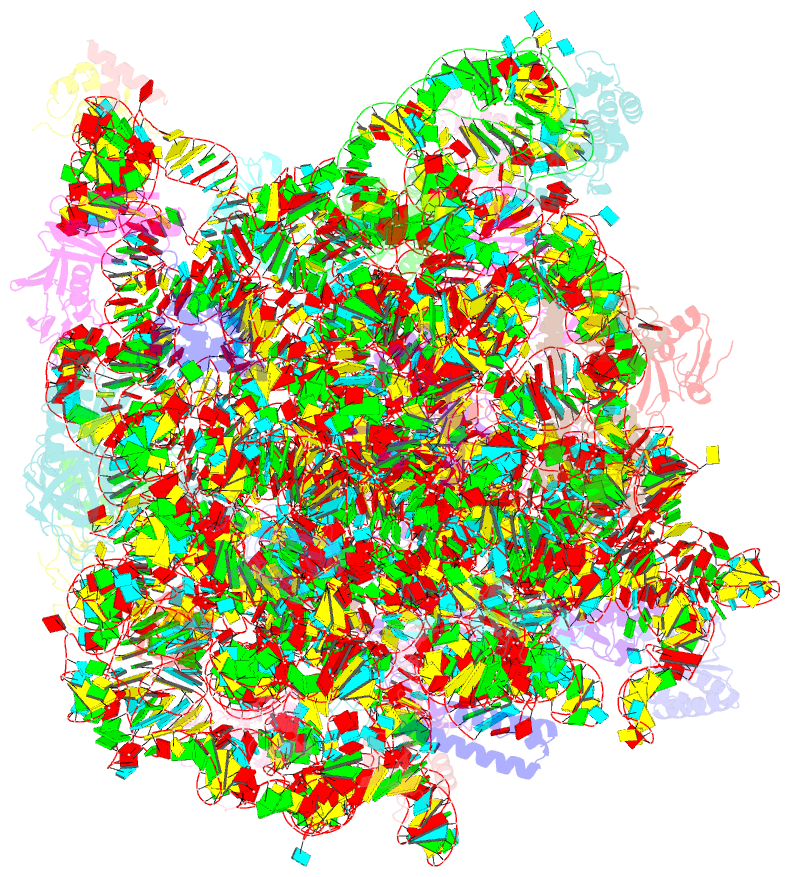
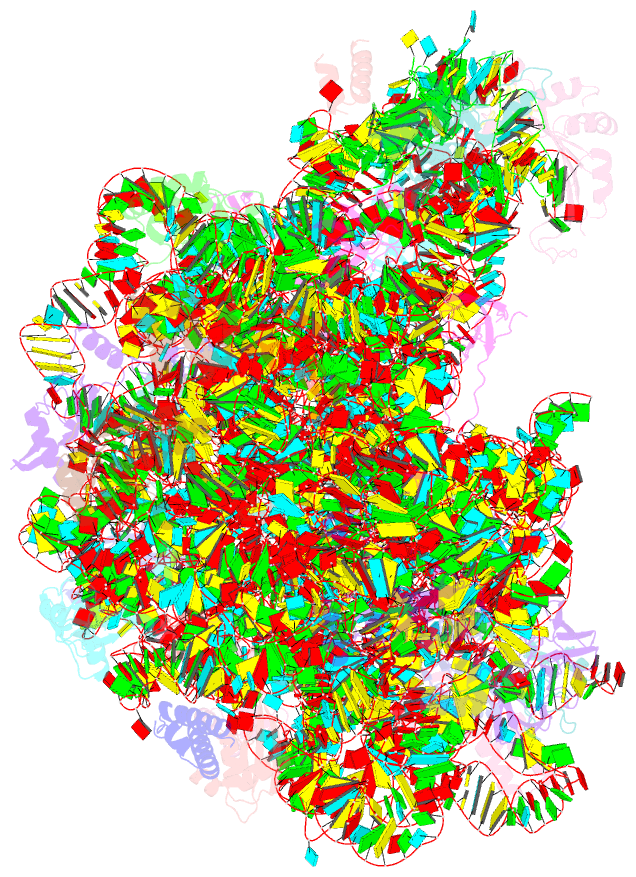
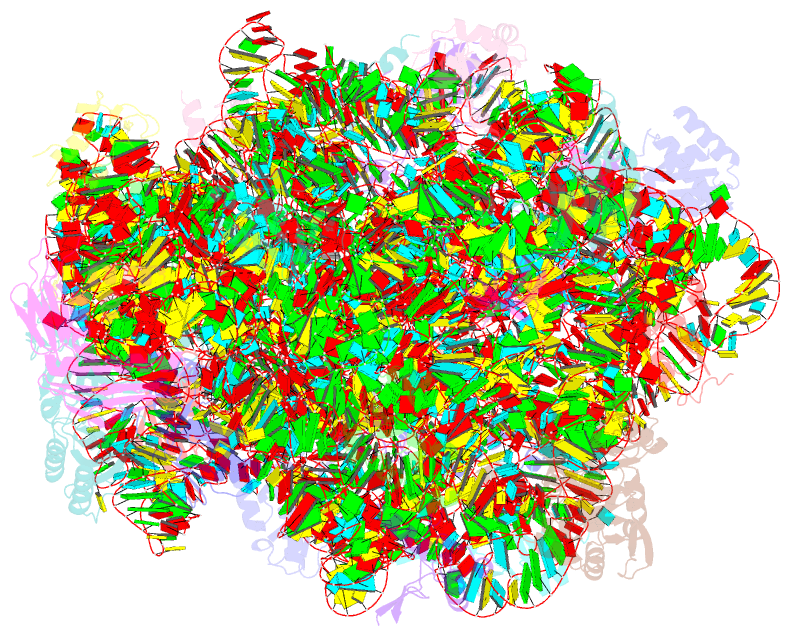
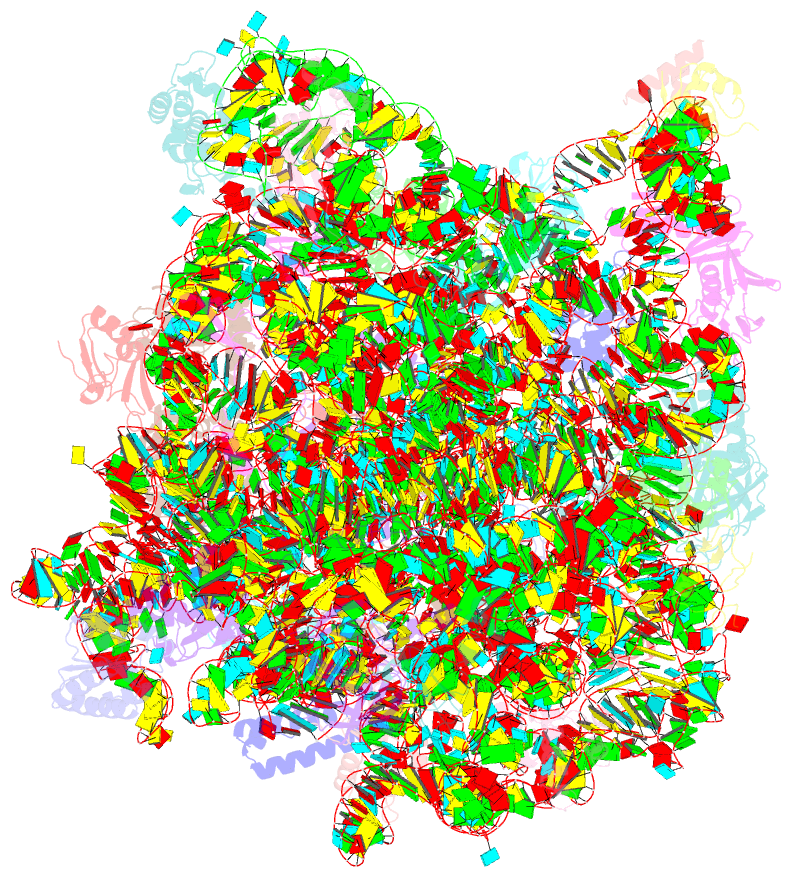
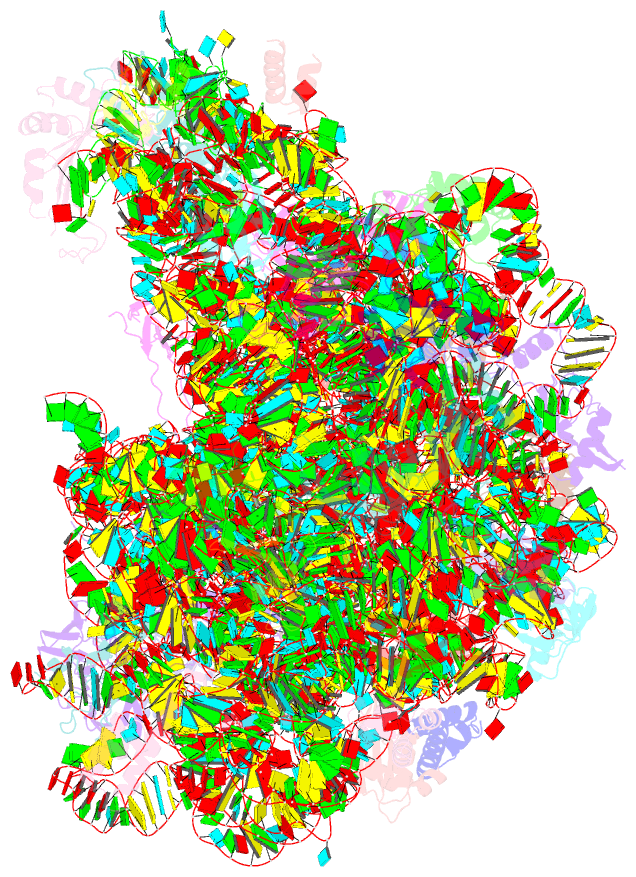
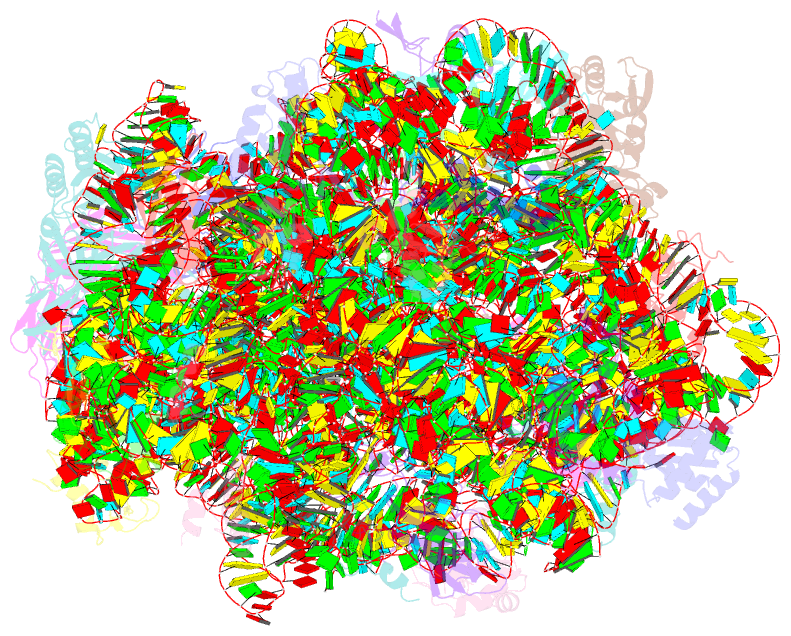
Summary information and primary citation
- PDB-id
-
3ccs;
DSSR-derived features in text and
JSON formats; DNAproDB
- Class
- ribosome
- Method
- X-ray (2.95 Å)
- Summary
- Structure of anisomycin resistant 50s ribosomal
subunit: 23s rrna mutation g2482a
- Reference
-
Blaha G, Gurel G, Schroeder SJ, Moore PB, Steitz TA
(2008): "Mutations
outside the anisomycin-binding site can make ribosomes
drug-resistant." J.Mol.Biol.,
379, 505-519. doi: 10.1016/j.jmb.2008.03.075.
- Abstract
- Eleven mutations that make Haloarcula marismortui
resistant to anisomycin, an antibiotic that competes with
the amino acid side chains of aminoacyl tRNAs for binding
to the A-site cleft of the large ribosomal unit, have been
identified in 23S rRNA. The correlation observed between
the sensitivity of H. marismortui to anisomycin and the
affinity of its large ribosomal subunits for the drug
indicates that its response to anisomycin is determined
primarily by the binding of the drug to its large ribosomal
subunit. The structures of large ribosomal subunits
containing resistance mutations show that these mutations
can be divided into two classes: (1) those that interfere
with specific drug-ribosome interactions and (2) those that
stabilize the apo conformation of the A-site cleft of the
ribosome relative to its drug-bound conformation. The
conformational effects of some mutations of the second kind
propagate through the ribosome for considerable distances
and are reversed when A-site substrates bind to the
ribosome.