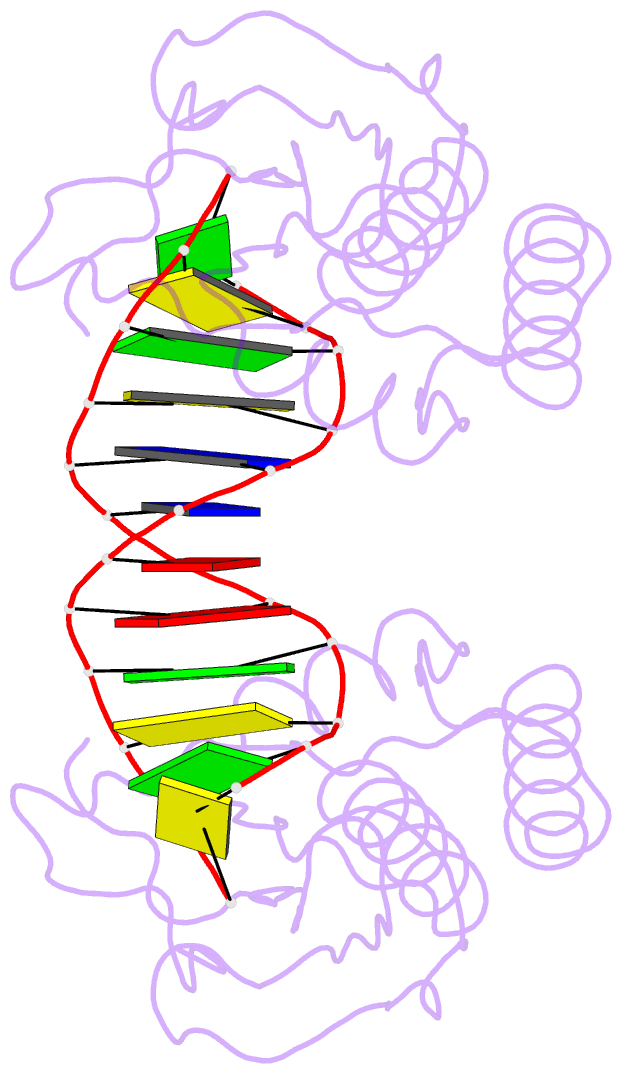
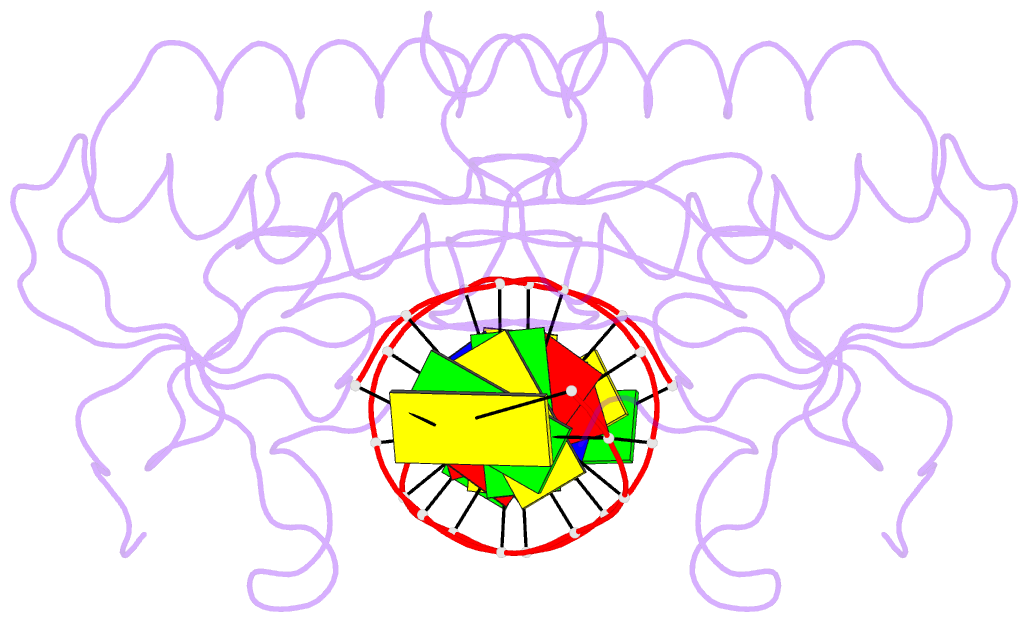
Summary information and primary citation
- PDB-id
- 3d0p; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (1.8 Å)
- Summary
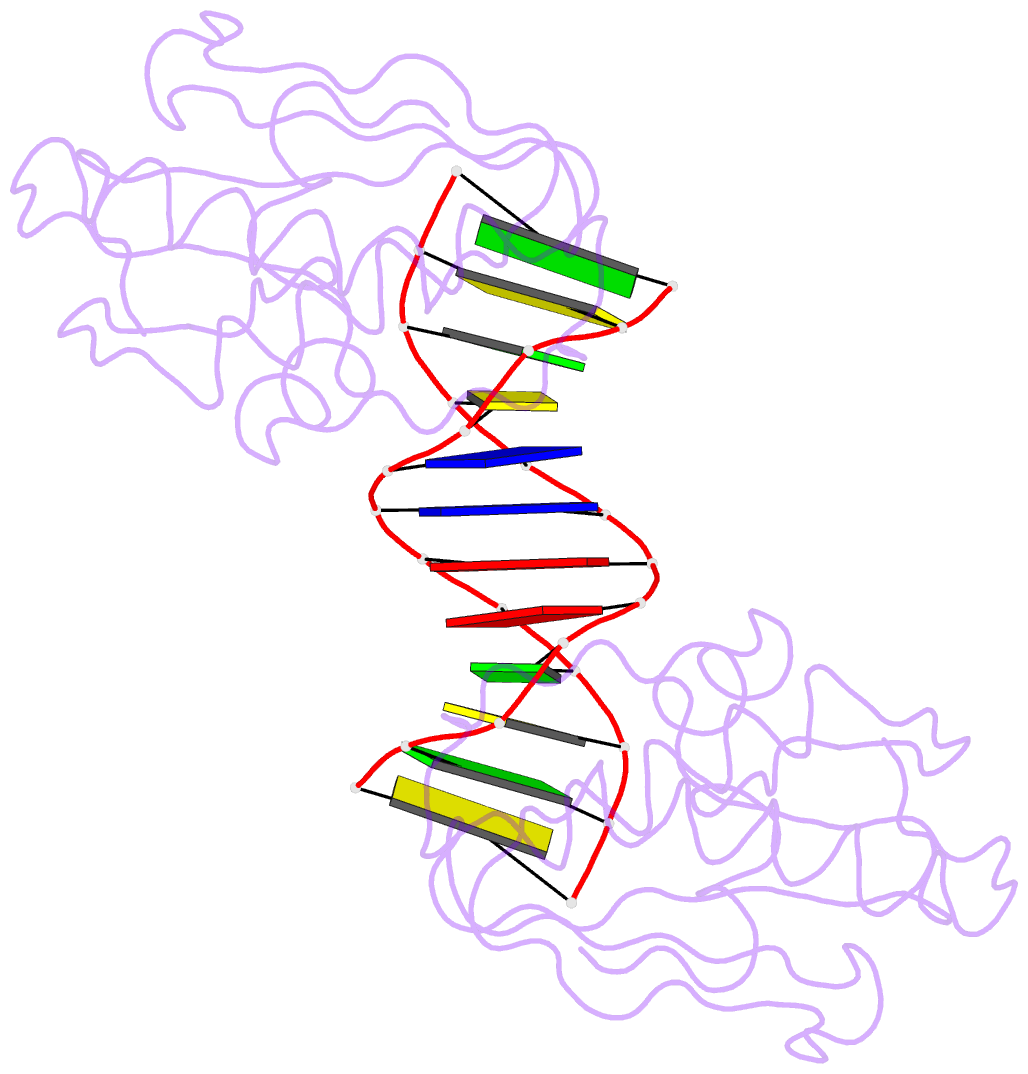
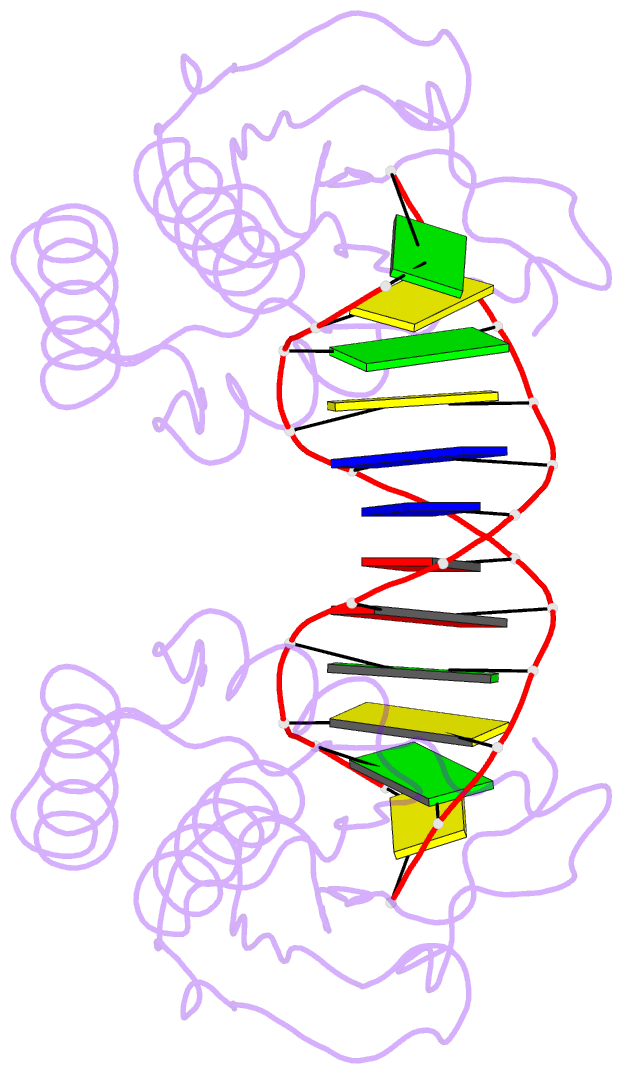
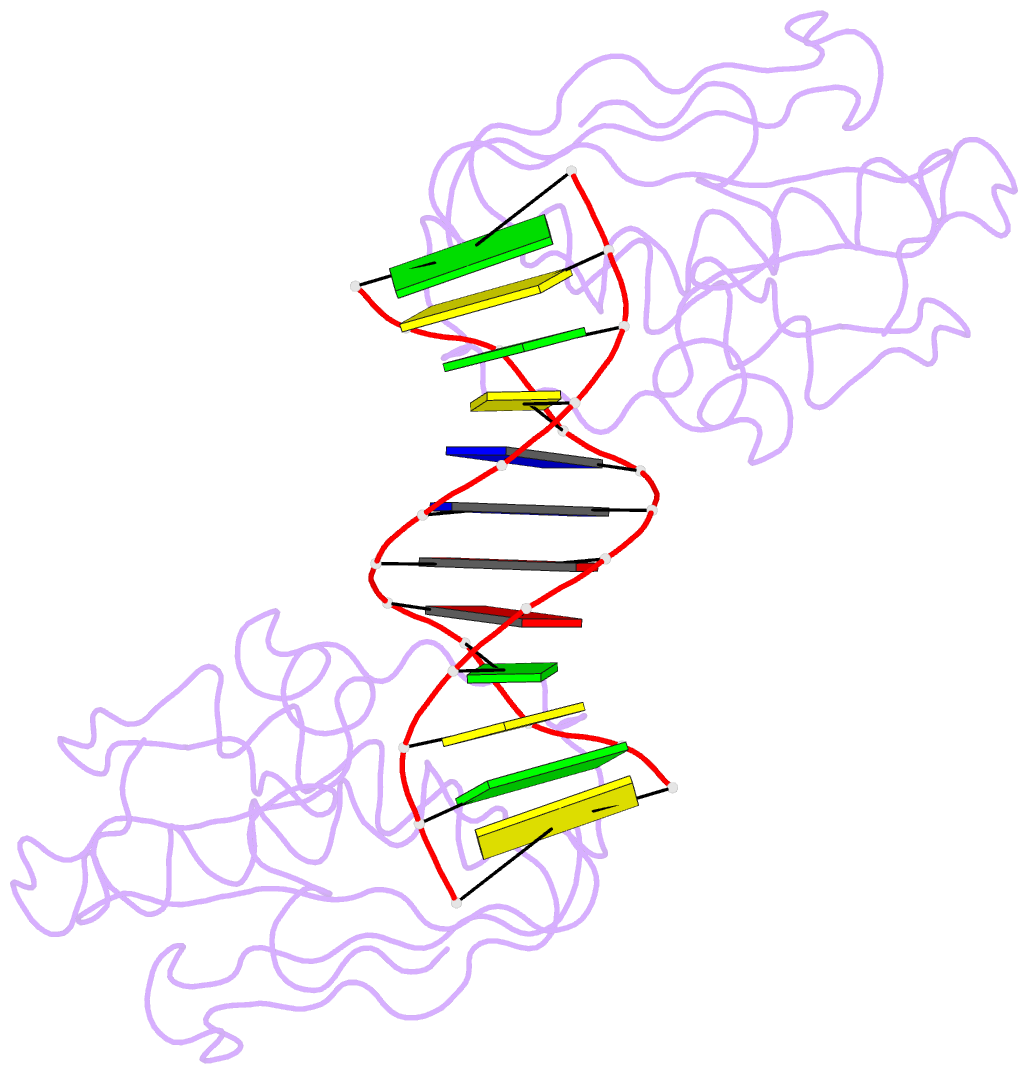
- Insights into RNA-DNA hybrid recognition and processing by rnase h from the crystal structure of a non-specific enzyme-dsDNA complex
- Reference
- Pallan PS, Egli M (2008): "Insights into RNA/DNA hybrid recognition and processing by RNase H from the crystal structure of a non-specific enzyme-dsDNA complex." Cell Cycle, 7, 2562-2569.
- Abstract
- Ribonuclease HI (RNase H) is a member of the nucleotidyl-transferase superfamily and endo-nucleolytically cleaves the RNA portion in RNA/DNA hybrids and removes RNA primers from Okazaki fragments. The enzyme also binds RNA and DNA duplexes but is unable to cleave either. Three-dimensional structures of bacterial and human RNase H catalytic domains bound to RNA/DNA hybrids have revealed the basis for substrate recognition and the mechanism of cleavage. In order to visualize the enzyme's interactions with duplex DNA and to establish the structural differences that afford tighter binding to RNA/DNA hybrids relative to dsDNA, we have determined the crystal structure of Bacillus halodurans RNase H in complex with the B-form DNA duplex [d(CGCGAATTCGCG)](2). The structure demonstrates that the inability of the enzyme to cleave DNA is due to the deviating curvature of the DNA strand relative to the substrate RNA strand and the absence of Mg(2+) at the active site. A subset of amino acids engaged in contacts to RNA 2'-hydroxyl groups in the substrate complex instead bind to bridging or non-bridging phosphodiester oxygens in the complex with dsDNA. Qualitative comparison of the enzyme's interactions with the substrate and inhibitor duplexes is consistent with the reduced binding affinity for the latter and sheds light on determinants of RNase H binding and cleavage specificity.