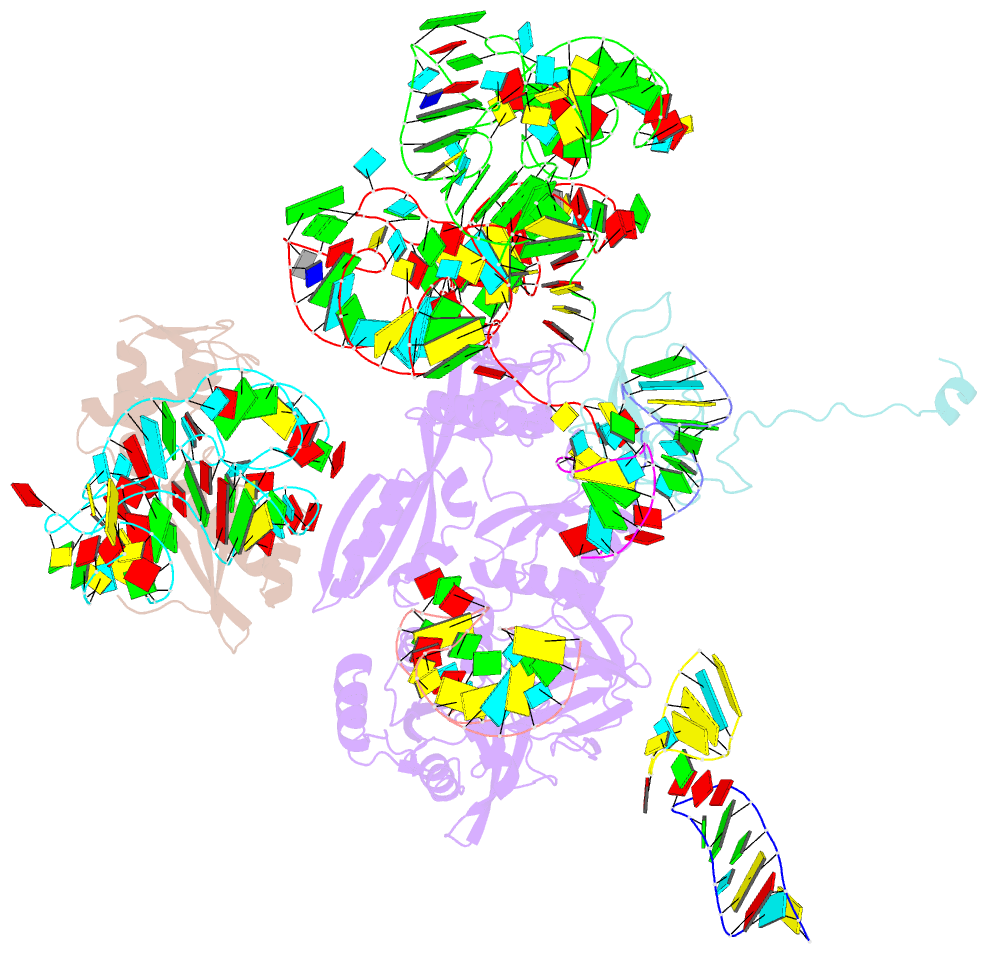
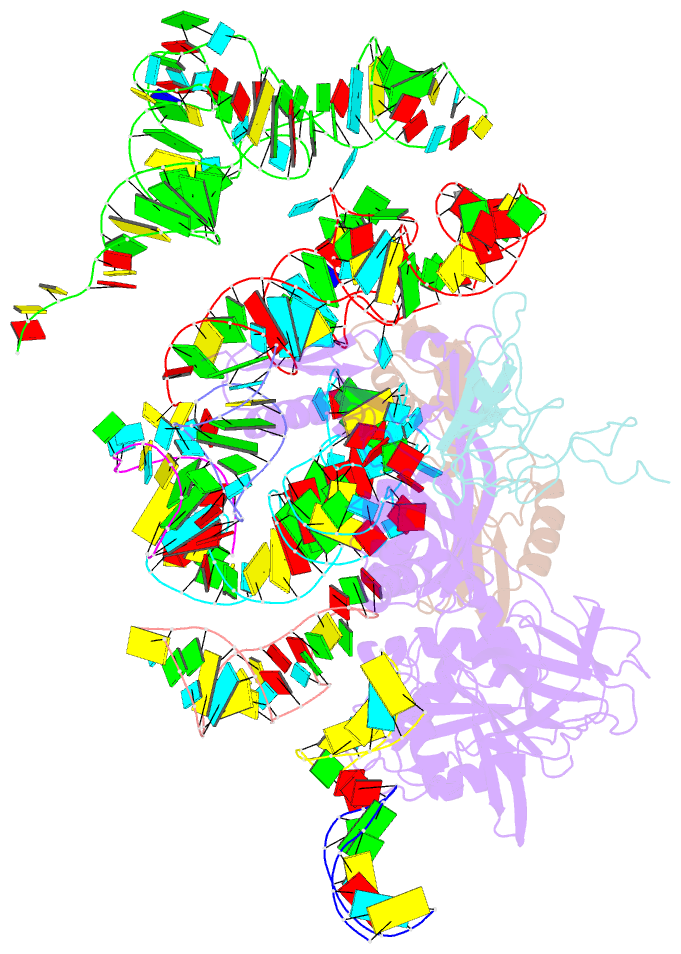
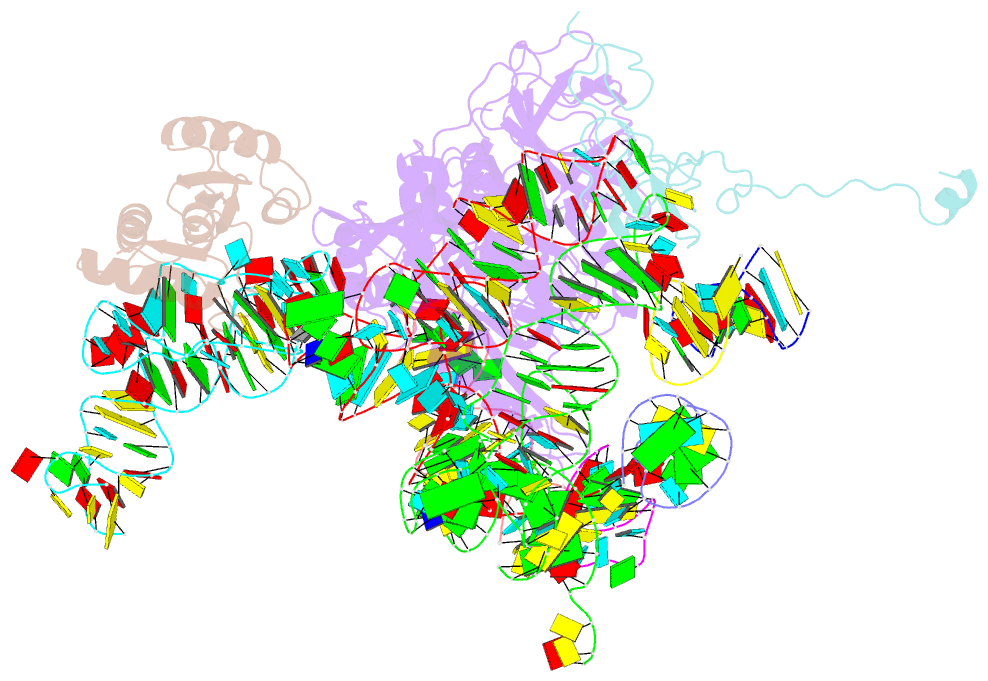
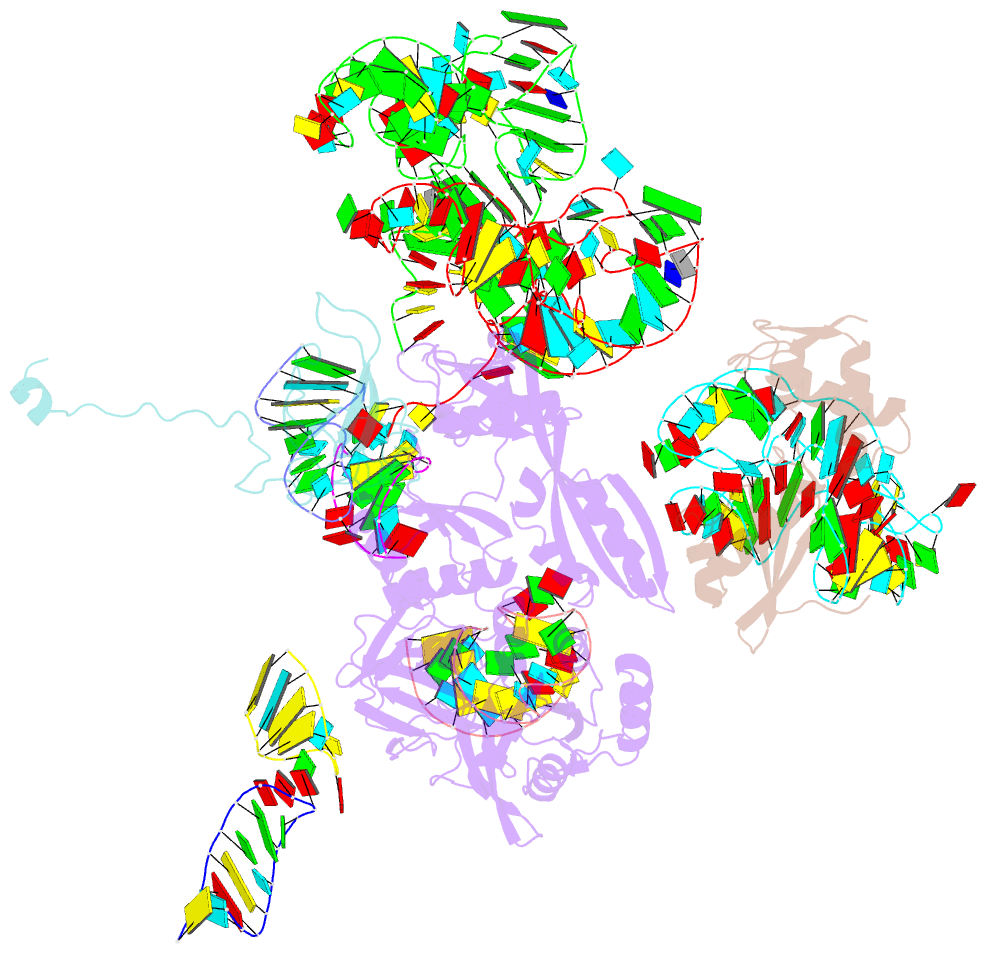
Summary information and primary citation
- PDB-id
- 3deg; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (10.9 Å)
- Summary
- Complex of elongating escherichia coli 70s ribosome and ef4(lepa)-gmppnp
- Reference
- Connell SR, Topf M, Qin Y, Wilson DN, Mielke T, Fucini P, Nierhaus KH, Spahn CMT (2008): "A new tRNA intermediate revealed on the ribosome during EF4-mediated back-translocation." Nat.Struct.Mol.Biol., 15, 910-915. doi: 10.1038/nsmb.1469.
- Abstract
- EF4 (LepA) is an almost universally conserved translational GTPase in eubacteria. It seems to be essential under environmental stress conditions and has previously been shown to back-translocate the tRNAs on the ribosome, thereby reverting the canonical translocation reaction. In the current work, EF4 was directly visualized in the process of back-translocating tRNAs by single-particle cryo-EM. Using flexible fitting methods, we built a model of ribosome-bound EF4 based on the cryo-EM map and a recently published unbound EF4 X-ray structure. The cryo-EM map establishes EF4 as a noncanonical elongation factor that interacts not only with the elongating ribosome, but also with the back-translocated tRNA in the A-site region, which is present in a previously unseen, intermediate state and deviates markedly from the position of a canonical A-tRNA. Our results, therefore, provide insight into the underlying structural principles governing back-translocation.