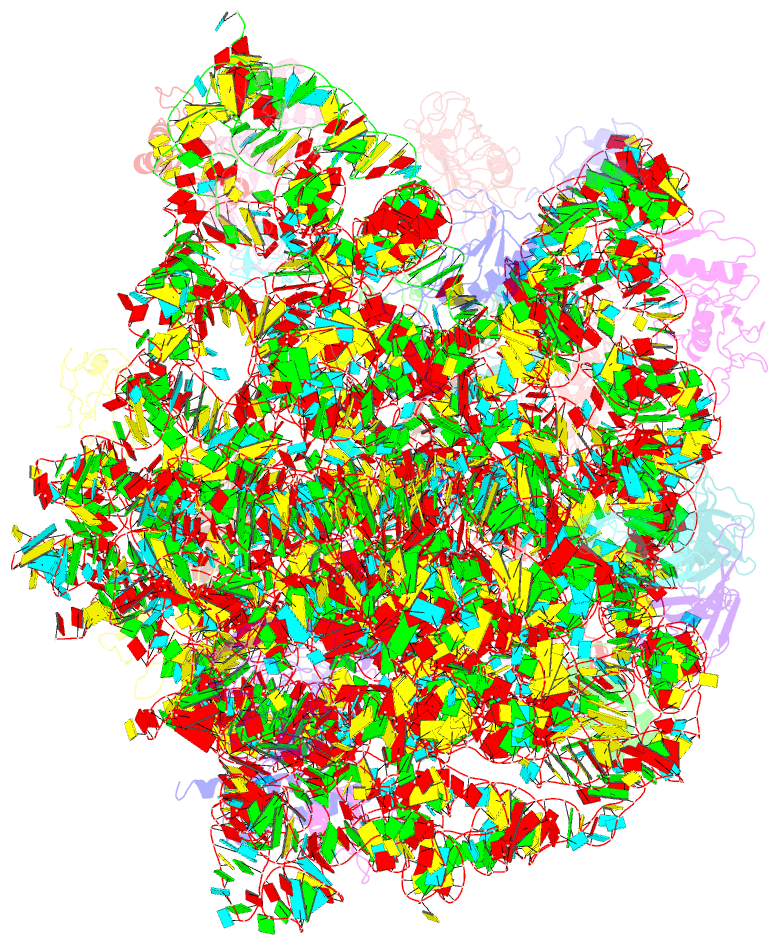
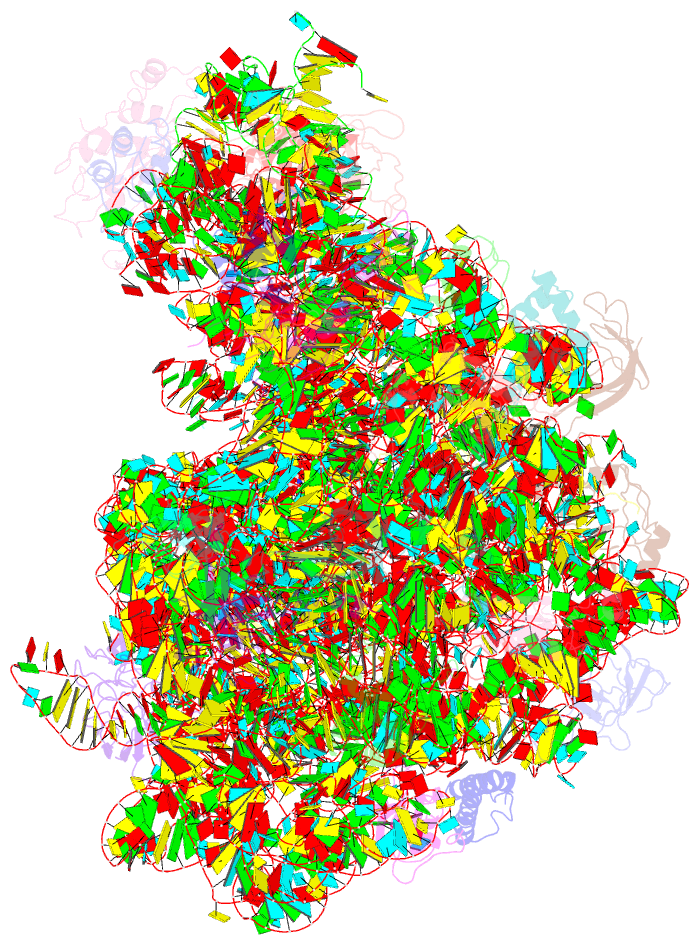
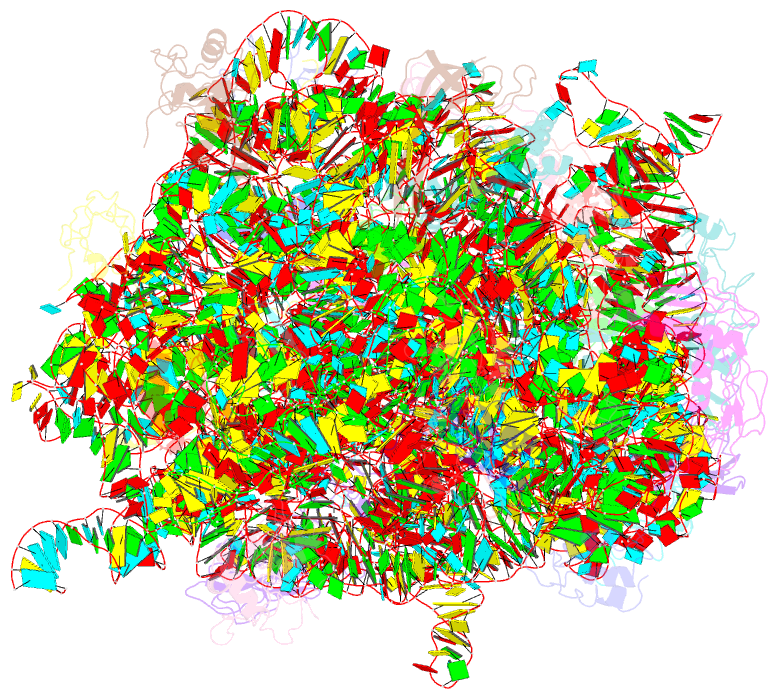
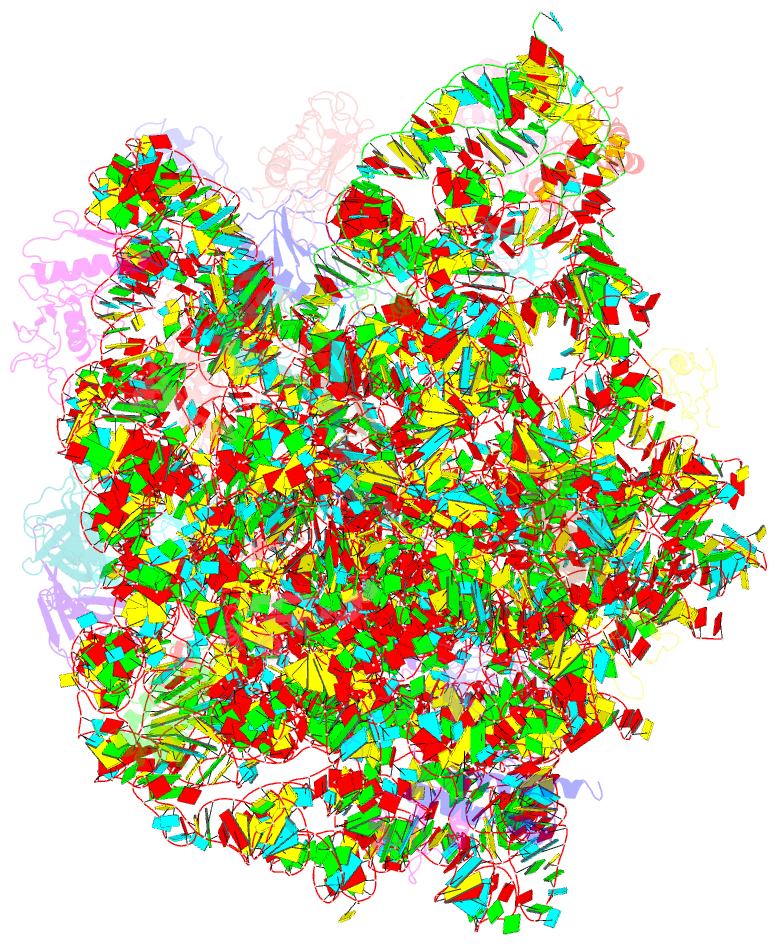
Summary information and primary citation
- PDB-id
- 3dll; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- X-ray (3.5 Å)
- Summary
- The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect trna positioning
- Reference
- Wilson DN, Schluenzen F, Harms JM, Starosta AL, Connell SR, Fucini P (2008): "The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning." Proc.Natl.Acad.Sci.Usa, 105, 13339-13344. doi: 10.1073/pnas.0804276105.
- Abstract
- The oxazolidinones represent the first new class of antibiotics to enter into clinical usage within the past 30 years, but their binding site and mechanism of action has not been fully characterized. We have determined the crystal structure of the oxazolidinone linezolid bound to the Deinococcus radiodurans 50S ribosomal subunit. Linezolid binds in the A site pocket at the peptidyltransferase center of the ribosome overlapping the aminoacyl moiety of an A-site bound tRNA as well as many clinically important antibiotics. Binding of linezolid stabilizes a distinct conformation of the universally conserved 23S rRNA nucleotide U2585 that would be nonproductive for peptide bond formation. In conjunction with available biochemical data, we present a model whereby oxazolidinones impart their inhibitory effect by perturbing the correct positioning of tRNAs on the ribosome.