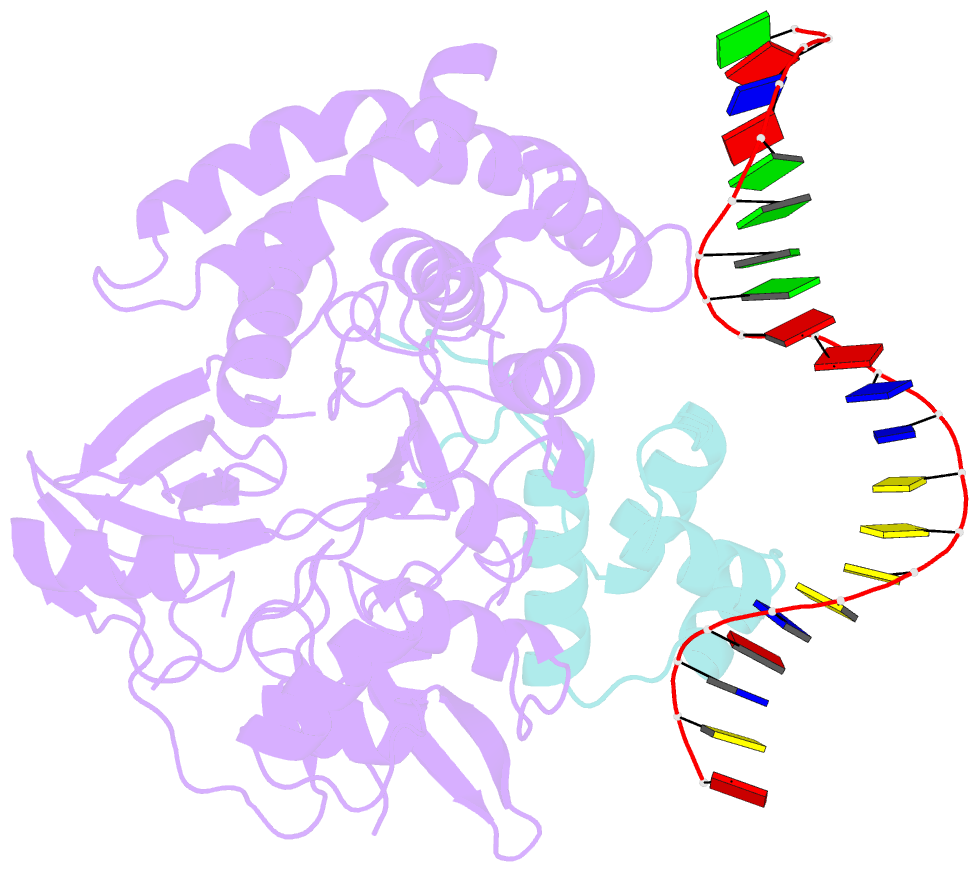
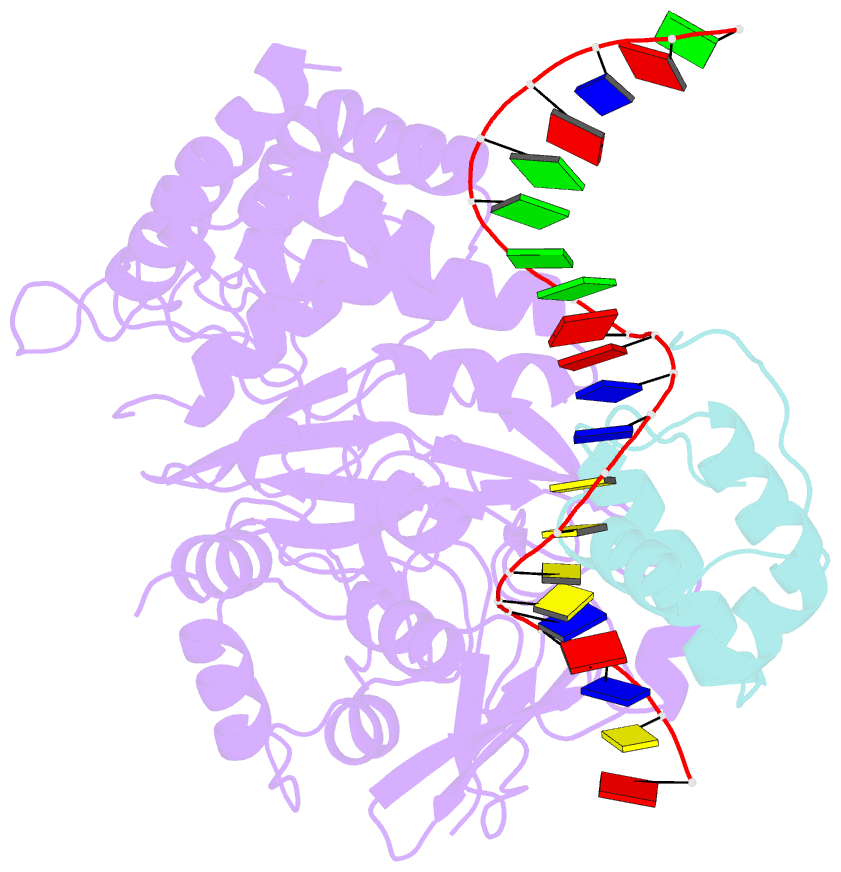
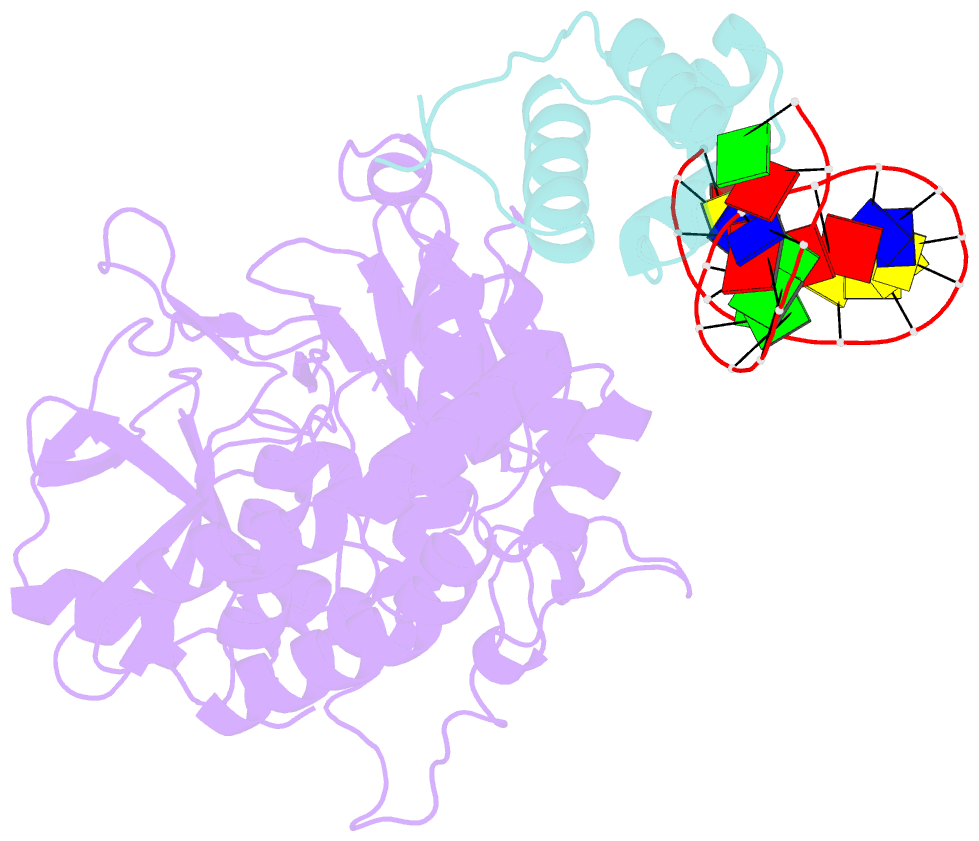
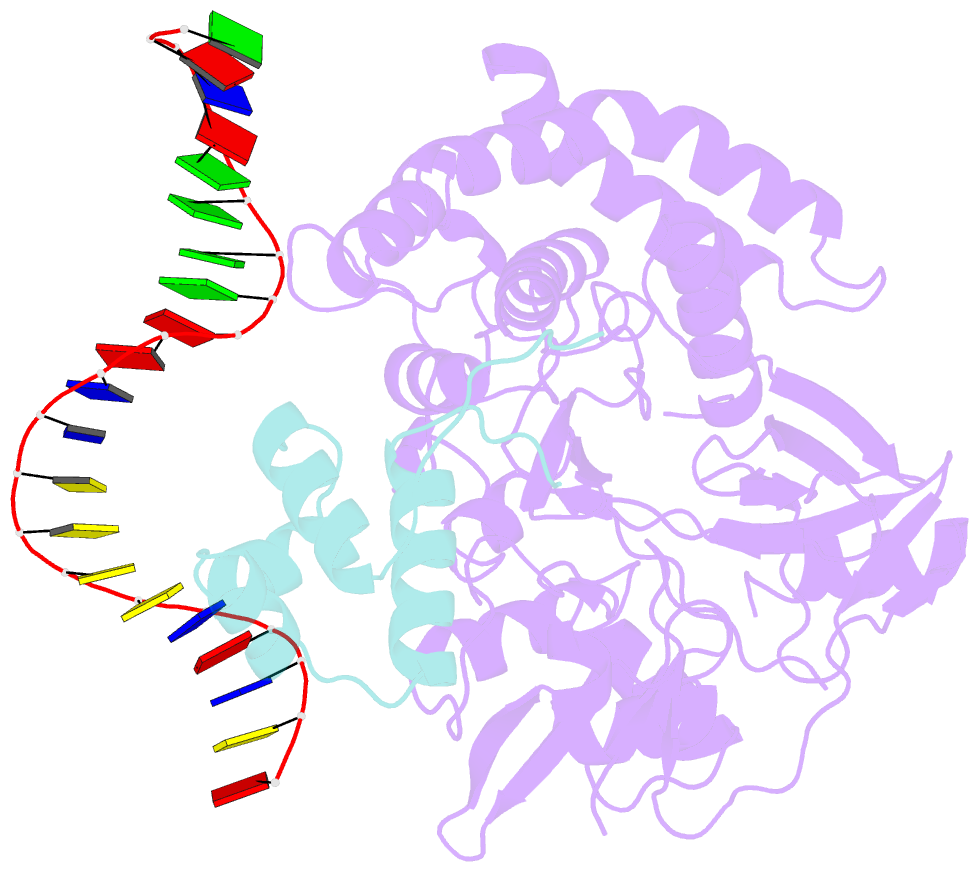
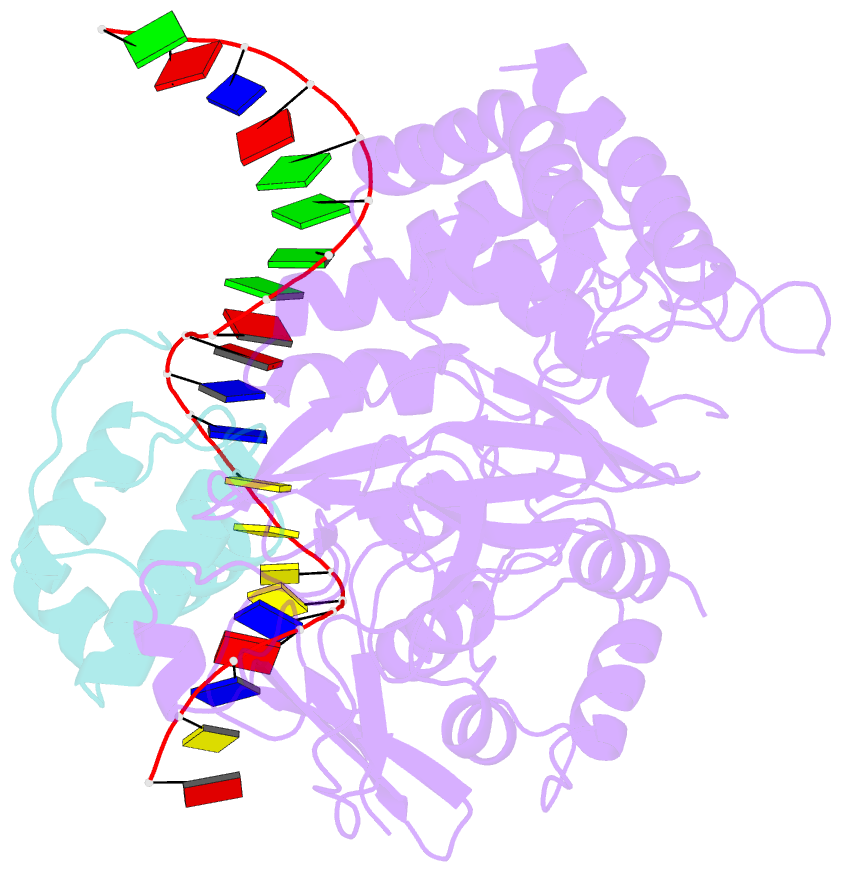
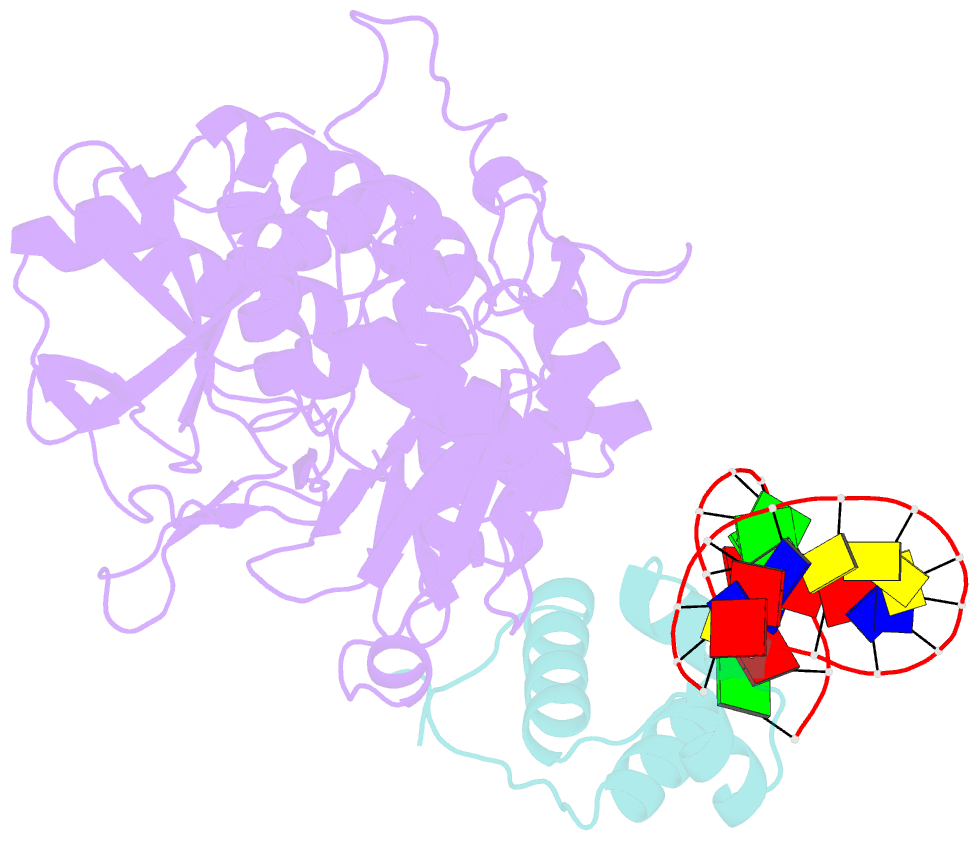
Summary information and primary citation
- PDB-id
- 3dnv; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.68 Å)
- Summary
- Mdt protein
- Reference
- Schumacher MA, Piro KM, Xu W, Hansen S, Lewis K, Brennan RG (2009): "Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB." Science, 323, 396-401. doi: 10.1126/science.1163806.
- Abstract
- Bacterial multidrug tolerance is largely responsible for the inability of antibiotics to eradicate infections and is caused by a small population of dormant bacteria called persisters. HipA is a critical Escherichia coli persistence factor that is normally neutralized by HipB, a transcription repressor, which also regulates hipBA expression. Here, we report multiple structures of HipA and a HipA-HipB-DNA complex. HipA has a eukaryotic serine/threonine kinase-like fold and can phosphorylate the translation factor EF-Tu, suggesting a persistence mechanism via cell stasis. The HipA-HipB-DNA structure reveals the HipB-operator binding mechanism, approximately 70 degrees DNA bending, and unexpected HipA-DNA contacts. Dimeric HipB interacts with two HipA molecules to inhibit its kinase activity through sequestration and conformational inactivation. Combined, these studies suggest mechanisms for HipA-mediated persistence and its neutralization by HipB.