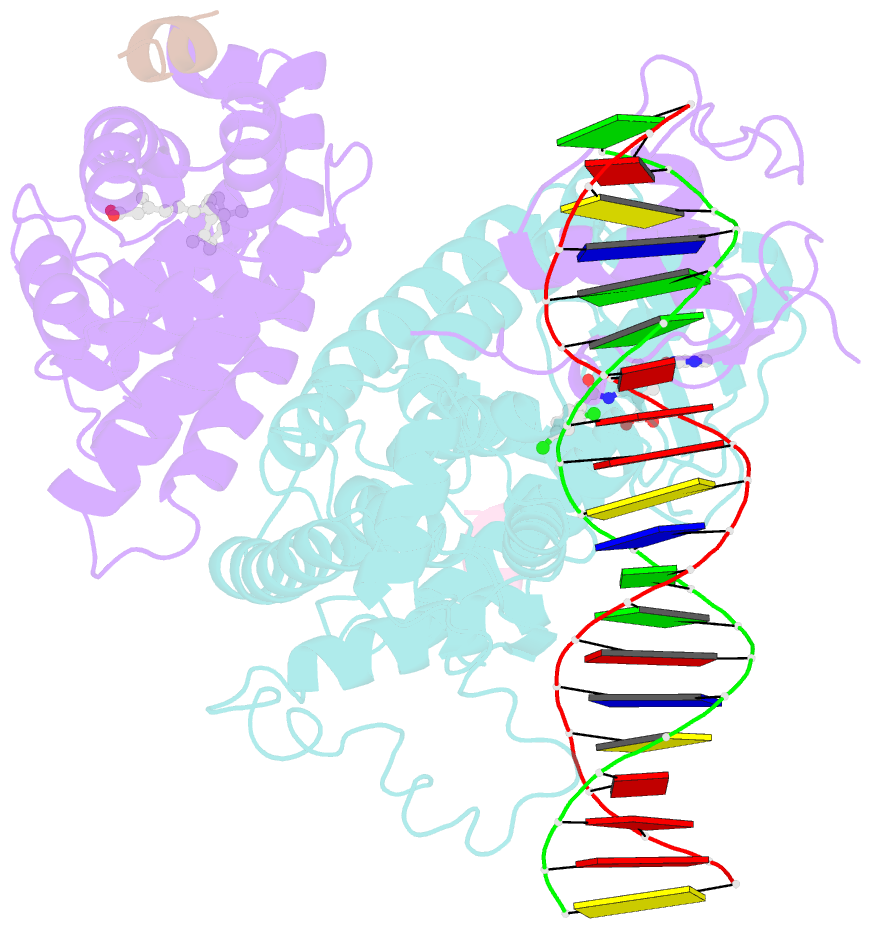
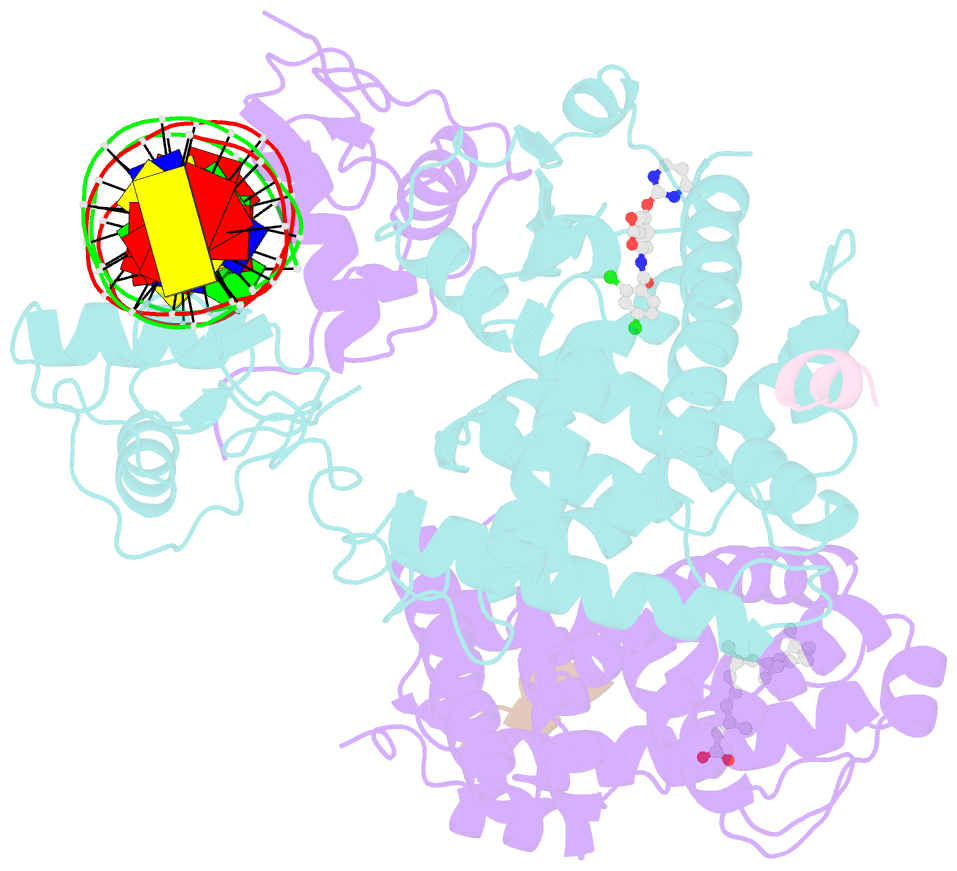
Summary information and primary citation
- PDB-id
- 3dzu; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (3.2 Å)
- Summary
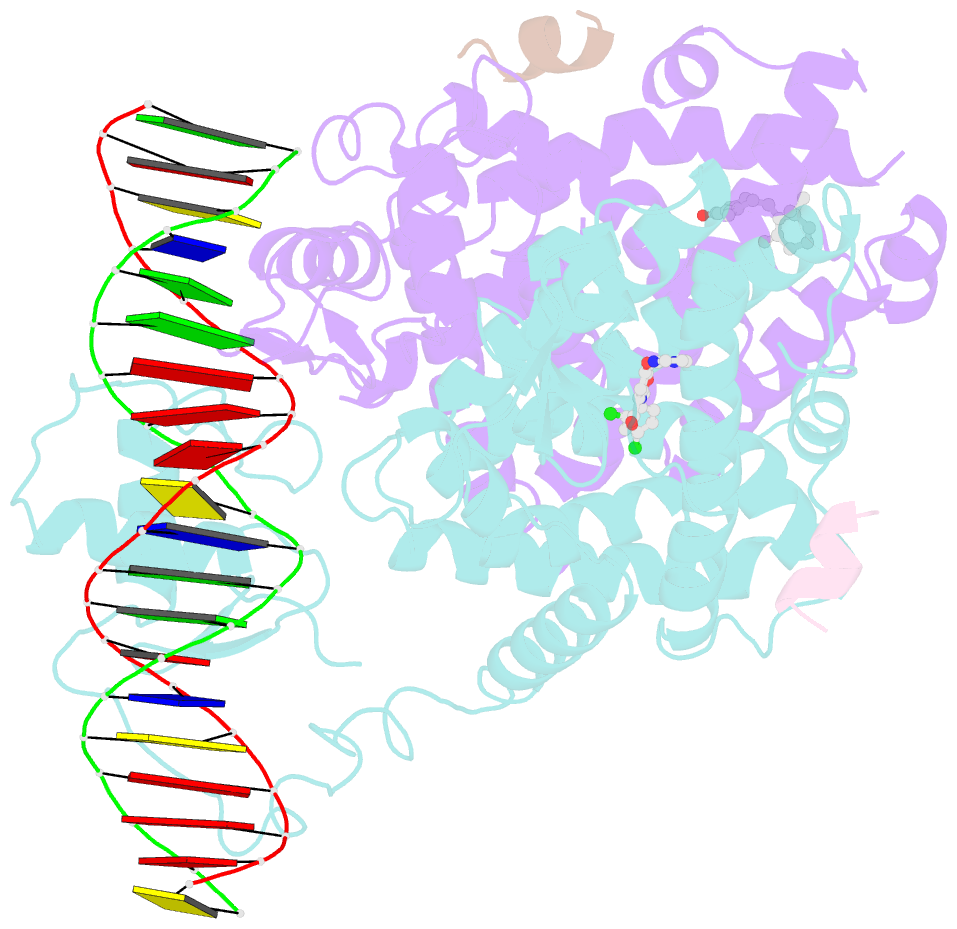
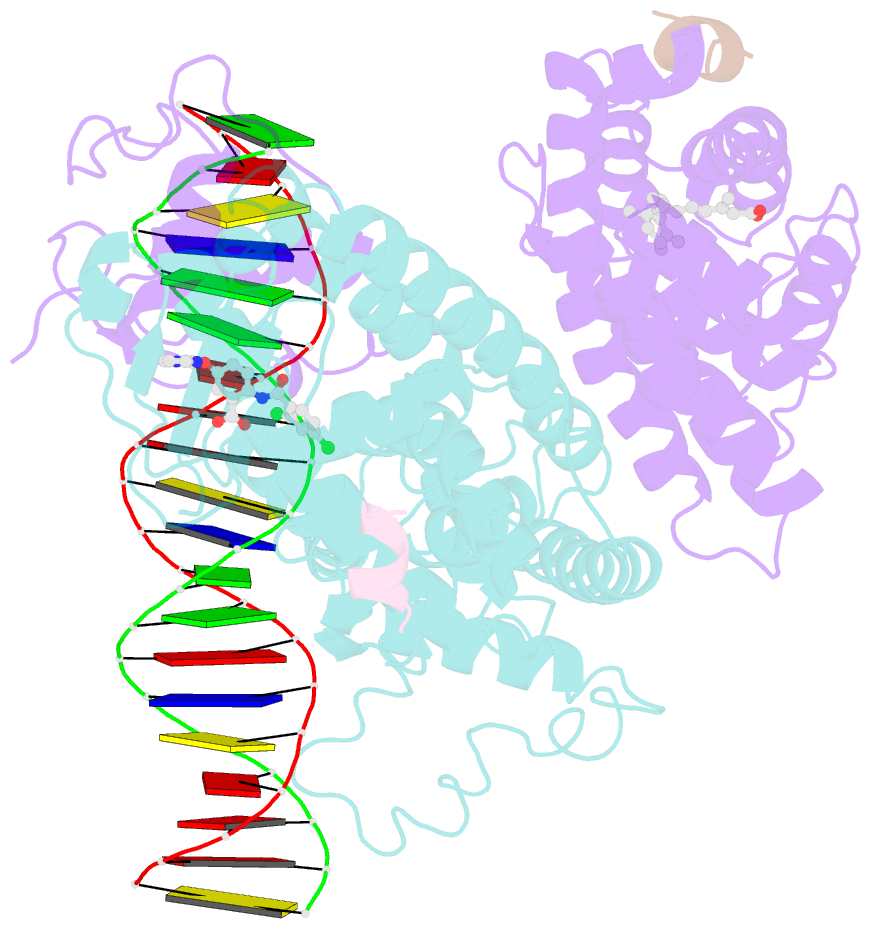
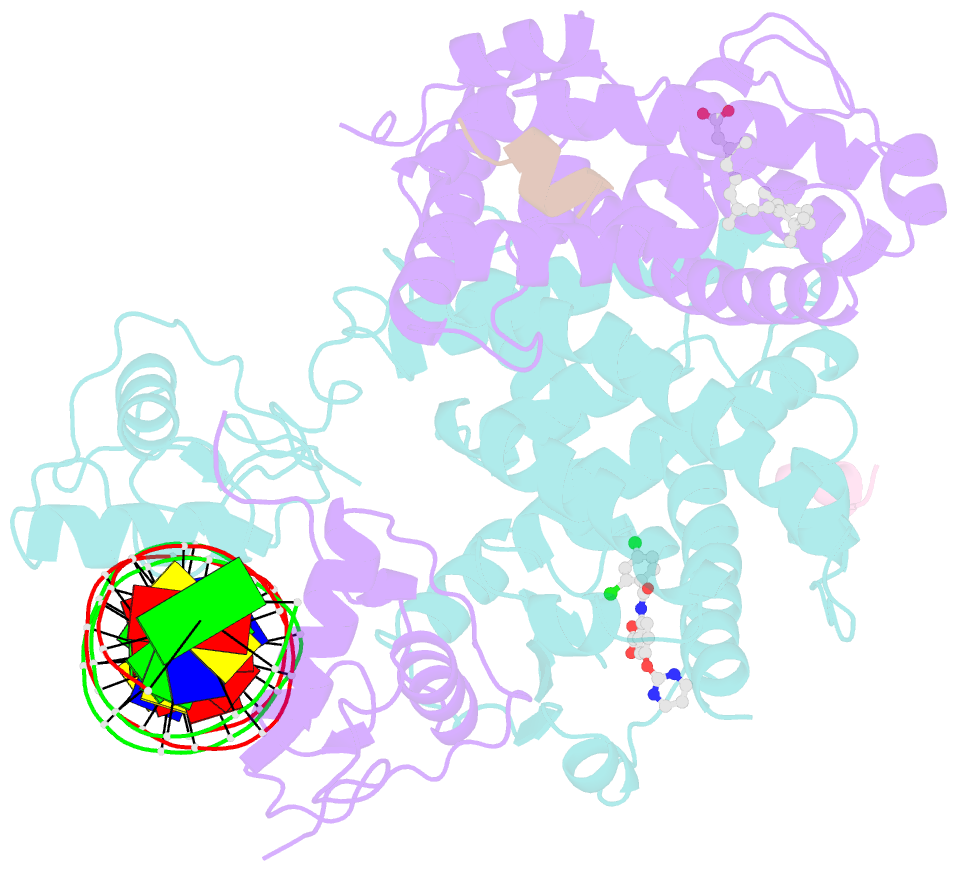
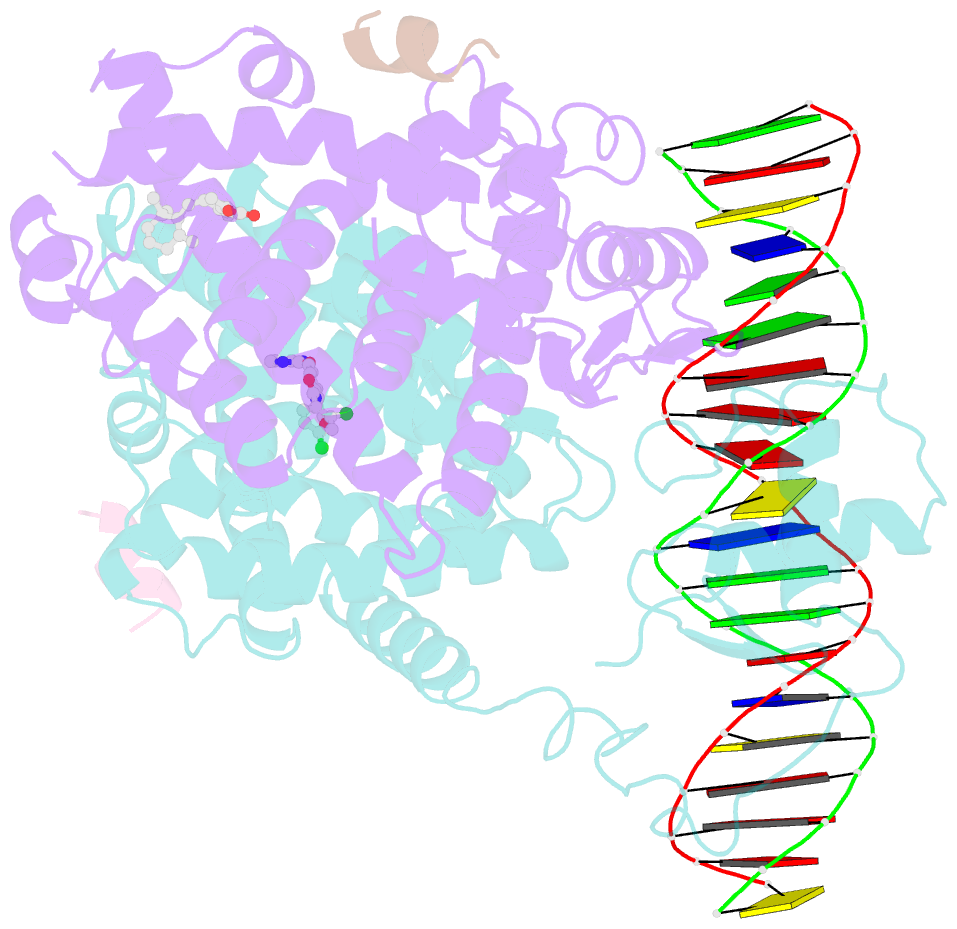
- Intact ppar gamma - rxr alpha nuclear receptor complex on DNA bound with bvt.13, 9-cis retinoic acid and ncoa2 peptide
- Reference
- Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F (2008): "Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA." Nature, 456, 350-356. doi: 10.1038/nature07413.
- Abstract
- Nuclear receptors are multi-domain transcription factors that bind to DNA elements from which they regulate gene expression. The peroxisome proliferator-activated receptors (PPARs) form heterodimers with the retinoid X receptor (RXR), and PPAR-gamma has been intensively studied as a drug target because of its link to insulin sensitization. Previous structural studies have focused on isolated DNA or ligand-binding segments, with no demonstration of how multiple domains cooperate to modulate receptor properties. Here we present structures of intact PPAR-gamma and RXR-alpha as a heterodimer bound to DNA, ligands and coactivator peptides. PPAR-gamma and RXR-alpha form a non-symmetric complex, allowing the ligand-binding domain (LBD) of PPAR-gamma to contact multiple domains in both proteins. Three interfaces link PPAR-gamma and RXR-alpha, including some that are DNA dependent. The PPAR-gamma LBD cooperates with both DNA-binding domains (DBDs) to enhance response-element binding. The A/B segments are highly dynamic, lacking folded substructures despite their gene-activation properties.