Summary information and primary citation
- PDB-id
- 3eqt; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (2.0 Å)
- Summary
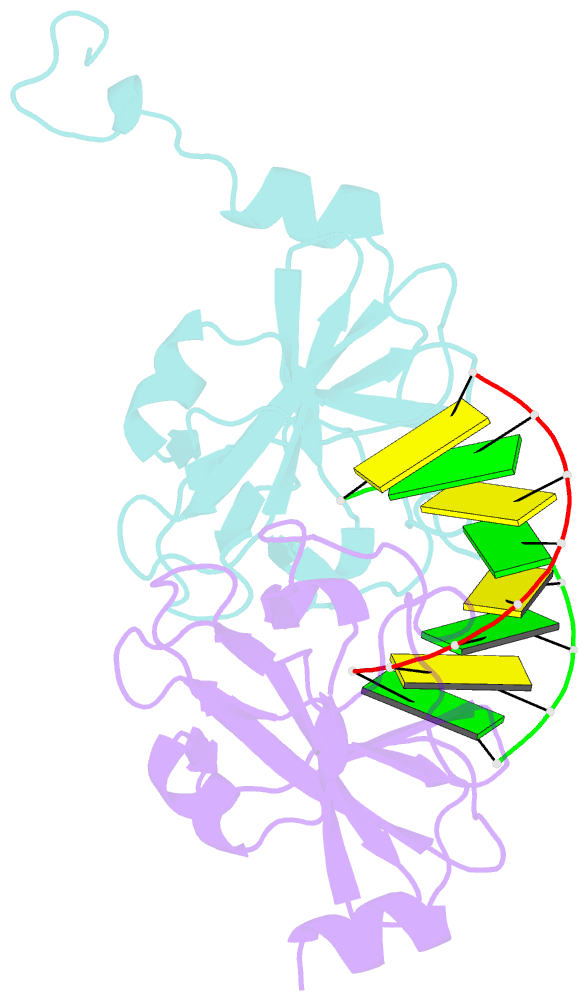
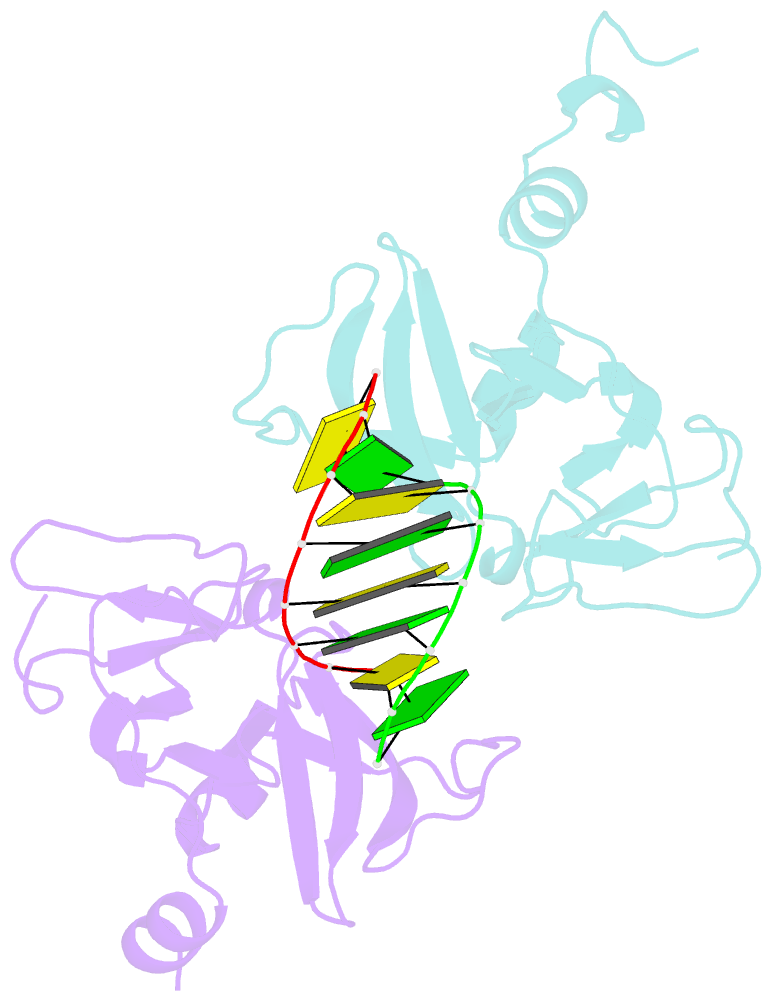
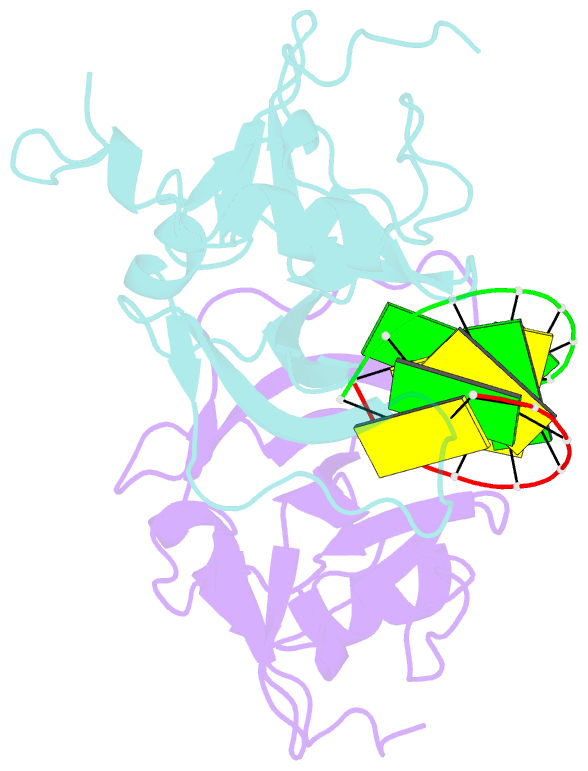
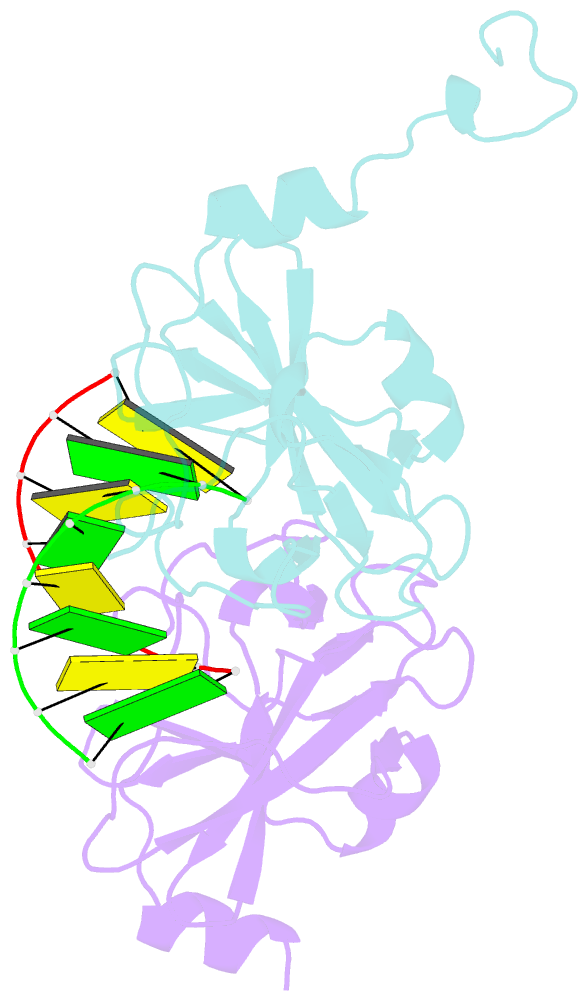
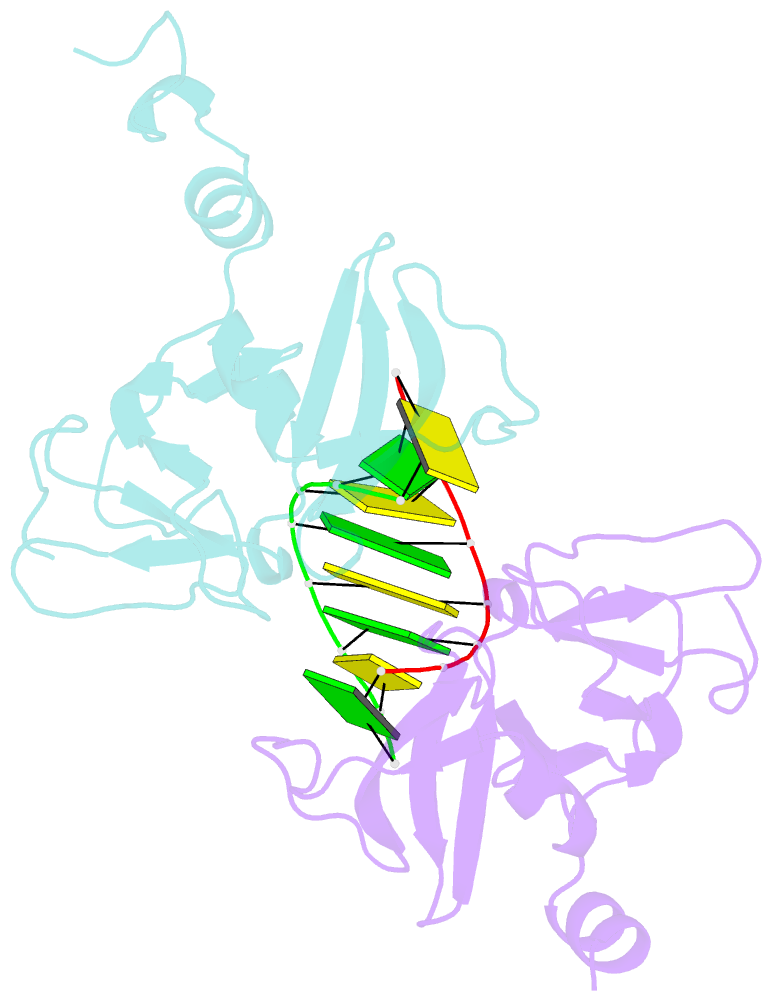
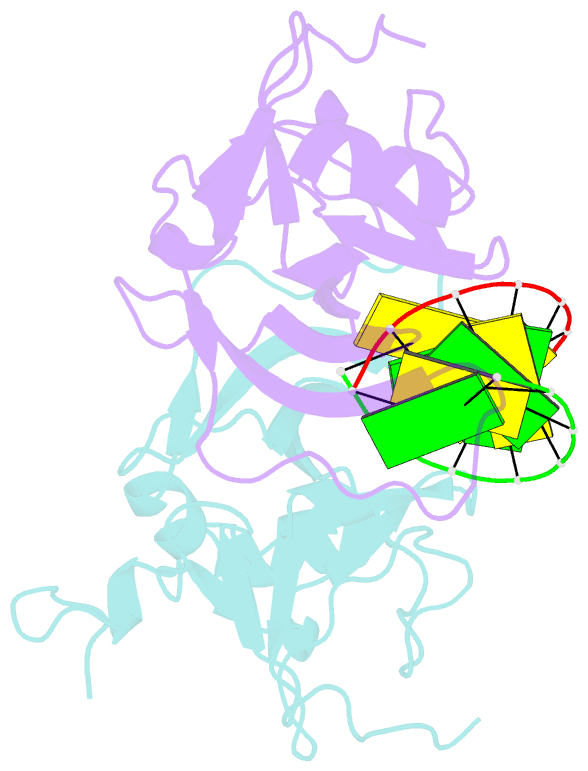
- Crystal structure of human lgp2 c-terminal domain in complex with dsrna
- Reference
- Li X, Ranjith-Kumar CT, Brooks MT, Dharmaiah S, Herr AB, Kao C, Li P (2009): "The RIG-I-like Receptor LGP2 Recognizes the Termini of Double-stranded RNA." J.Biol.Chem., 284, 13881-13891. doi: 10.1074/jbc.M900818200.
- Abstract
- The RIG-I-like receptors (RLRs), RIG-I and MDA5, recognize single-stranded RNA with 5' triphosphates and double-stranded RNA (dsRNA) to initiate innate antiviral immune responses. LGP2, a homolog of RIG-I and MDA5 that lacks signaling capability, regulates the signaling of the RLRs. To establish the structural basis of dsRNA recognition by the RLRs, we have determined the 2.0-A resolution crystal structure of human LGP2 C-terminal domain bound to an 8-bp dsRNA. Two LGP2 C-terminal domain molecules bind to the termini of dsRNA with minimal contacts between the protein molecules. Gel filtration chromatography and analytical ultracentrifugation demonstrated that LGP2 binds blunt-ended dsRNA of different lengths, forming complexes with 2:1 stoichiometry. dsRNA with protruding termini bind LGP2 and RIG-I weakly and do not stimulate the activation of RIG-I efficiently in cells. Surprisingly, full-length LGP2 containing mutations that abolish dsRNA binding retained the ability to inhibit RIG-I signaling.