Summary information and primary citation
- PDB-id
- 3g0h; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA
- Method
- X-ray (2.7 Å)
- Summary
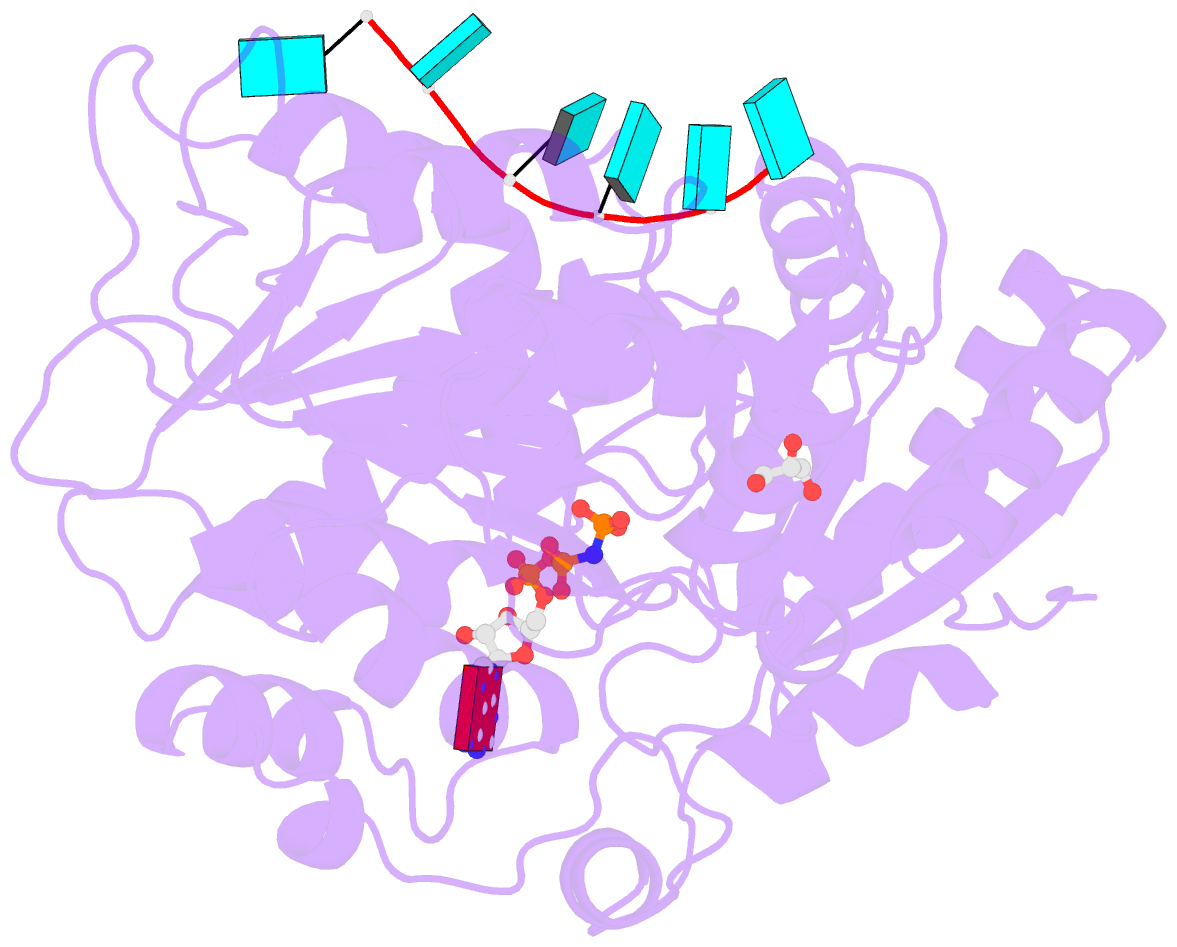
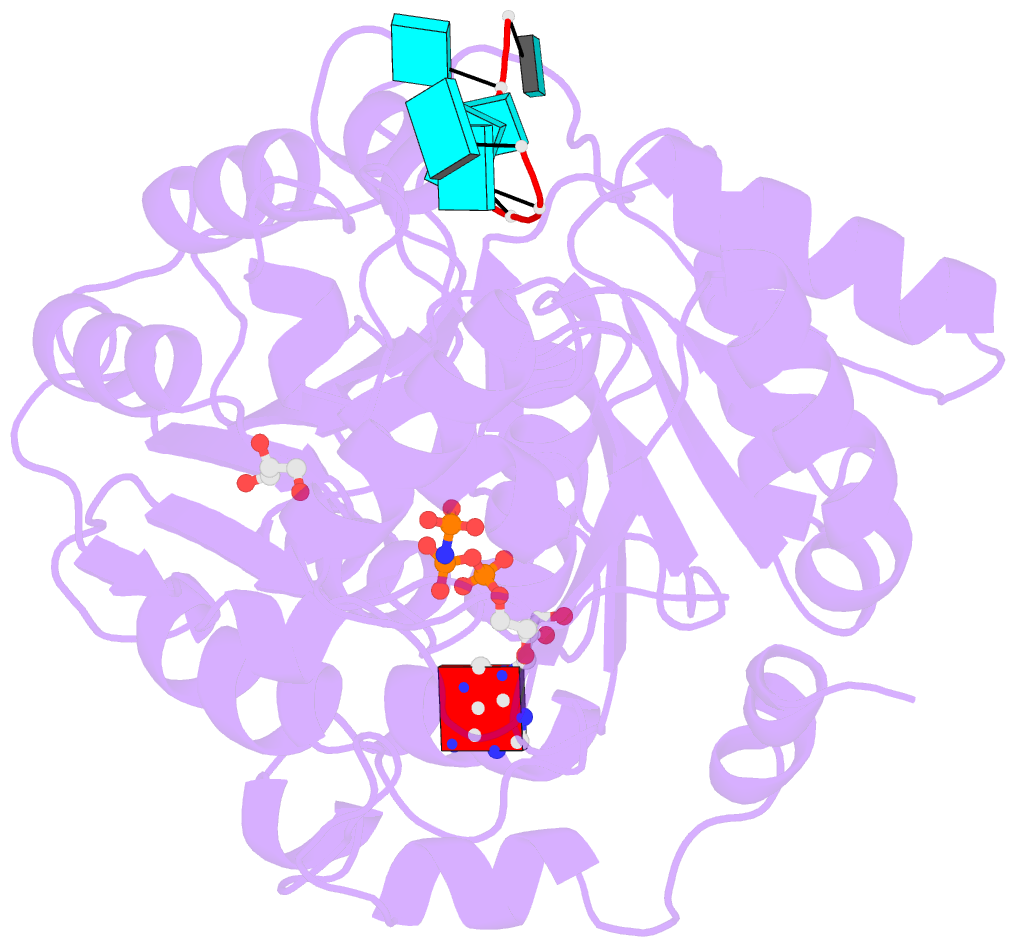
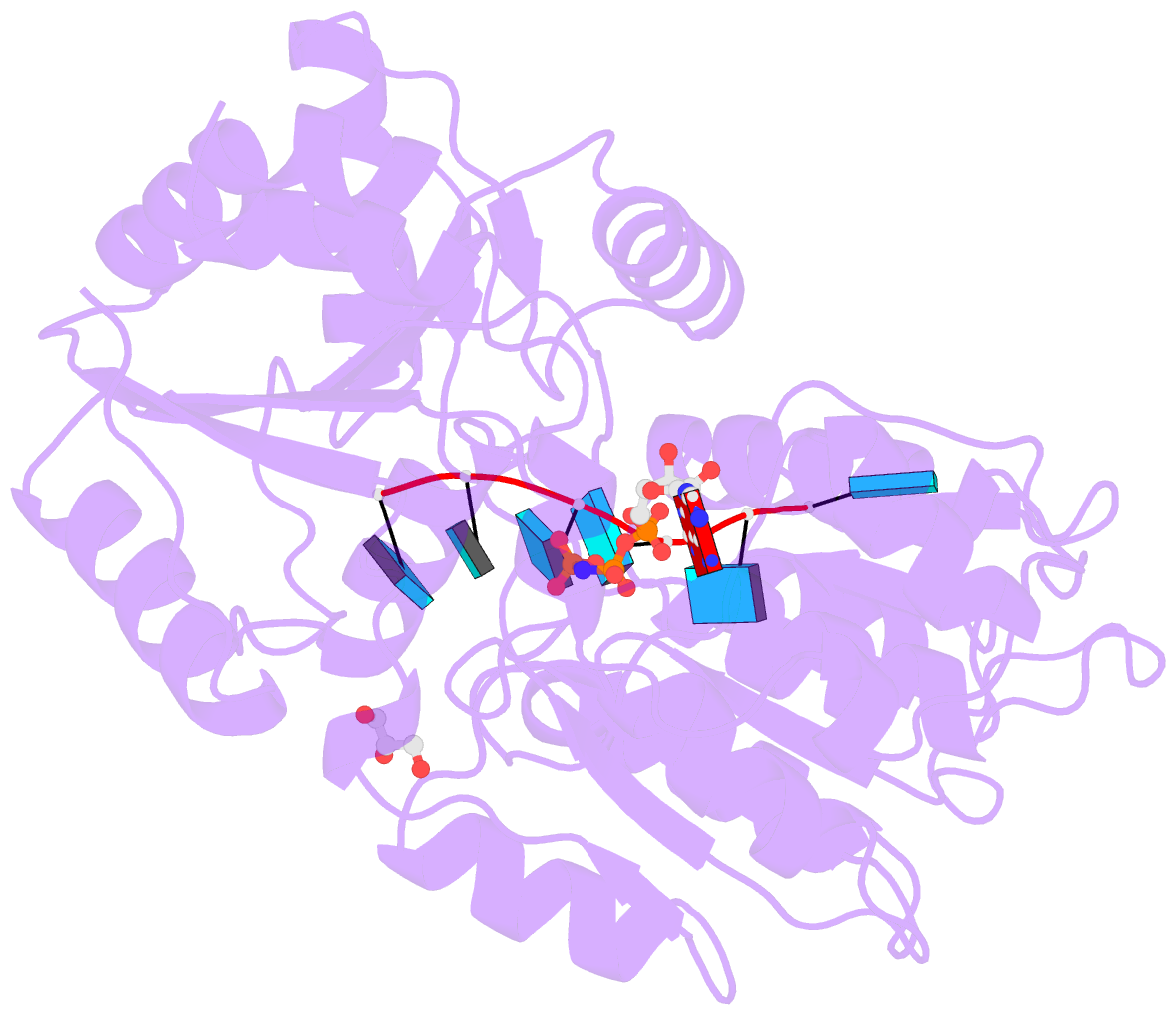
- Human dead-box RNA helicase ddx19, in complex with an atp-analogue and RNA
- Reference
- Collins R, Karlberg T, Lehtio L, Schutz P, van den Berg S, Dahlgren LG, Hammarstrom M, Weigelt J, Schuler H (2009): "The DEXD/H-box RNA Helicase DDX19 Is Regulated by an {alpha}-Helical Switch." J.Biol.Chem., 284, 10296-10300. doi: 10.1074/jbc.C900018200.
- Abstract
- DEXD/H-box RNA helicases couple ATP hydrolysis to RNA remodeling by an unknown mechanism. We used x-ray crystallography and biochemical analysis of the human DEXD/H-box protein DDX19 to investigate its regulatory mechanism. The crystal structures of DDX19, in its RNA-bound prehydrolysis and free posthydrolysis state, reveal an alpha-helix that inserts between the conserved domains of the free protein to negatively regulate ATPase activity. This finding was corroborated by biochemical data that confirm an autoregulatory function of the N-terminal region of the protein. This is the first study describing crystal structures of a DEXD/H-box protein in its open and closed cleft conformations.