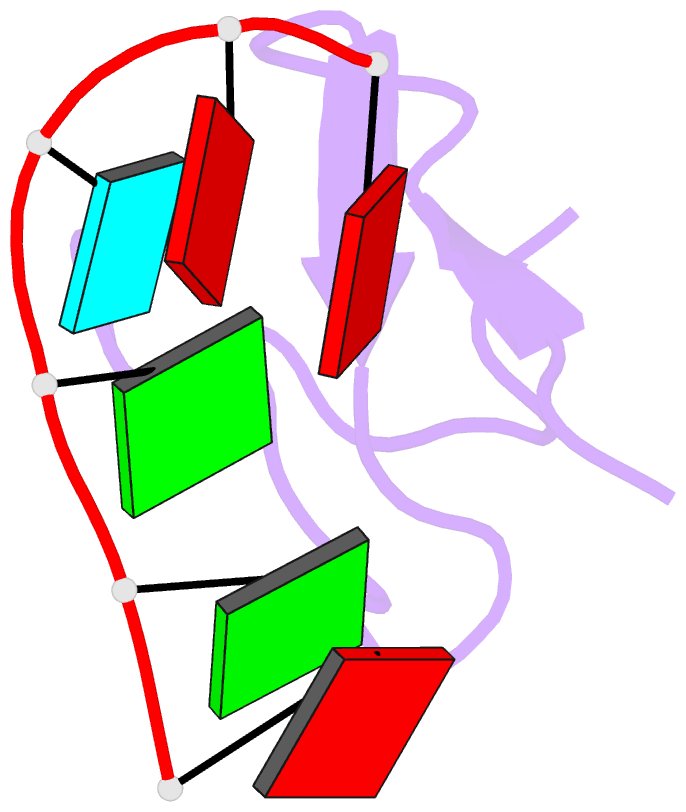
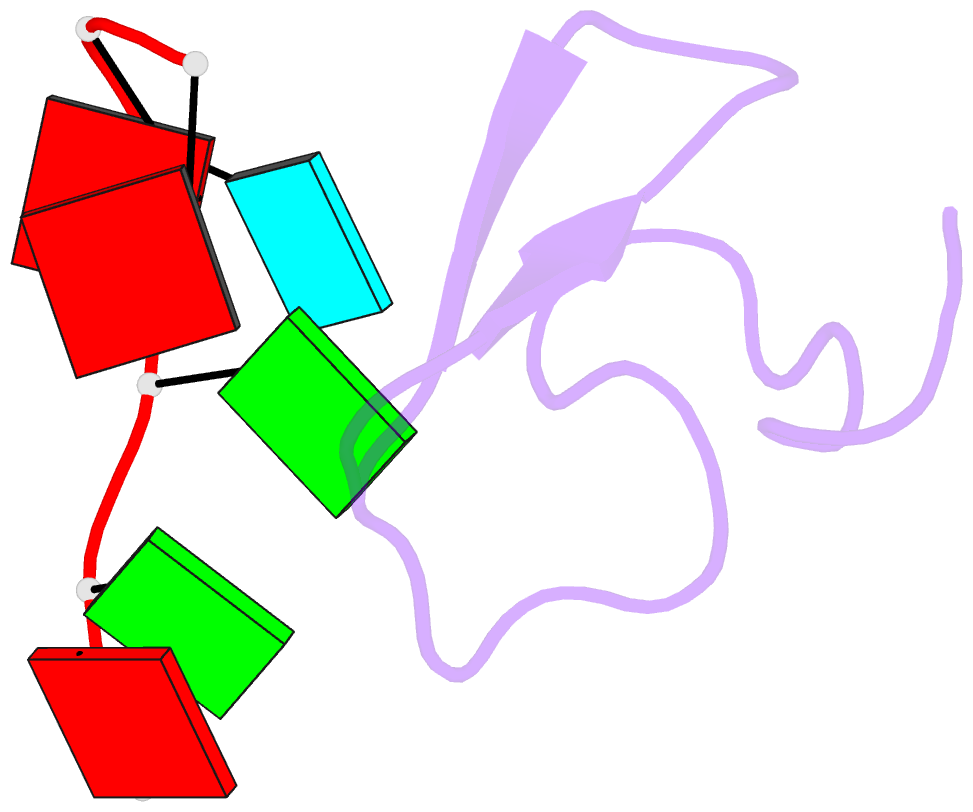
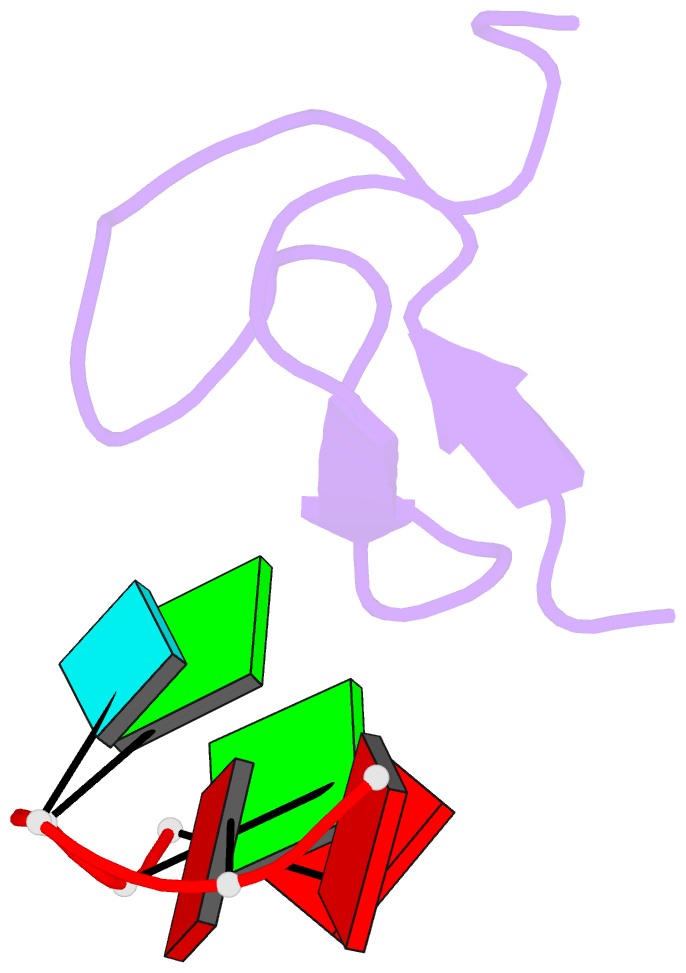
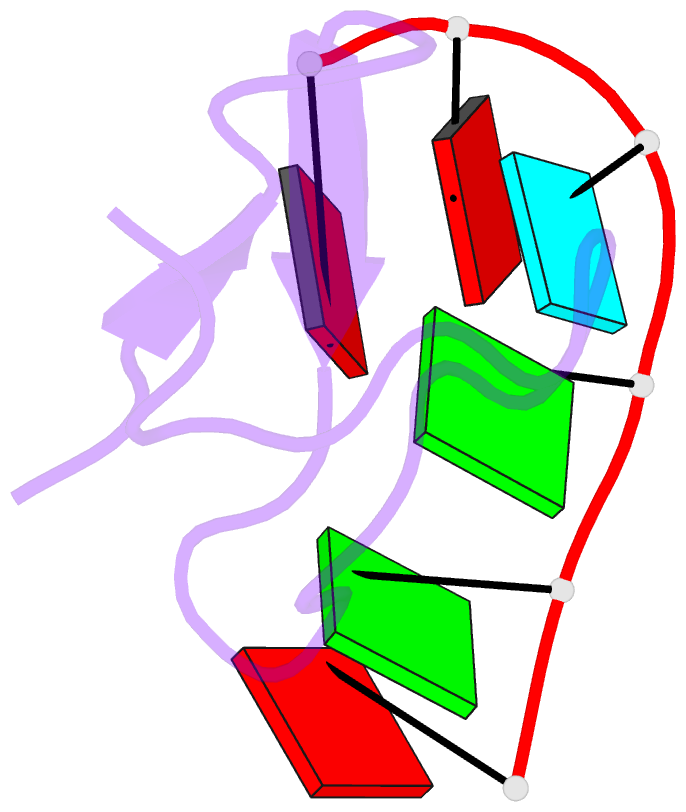
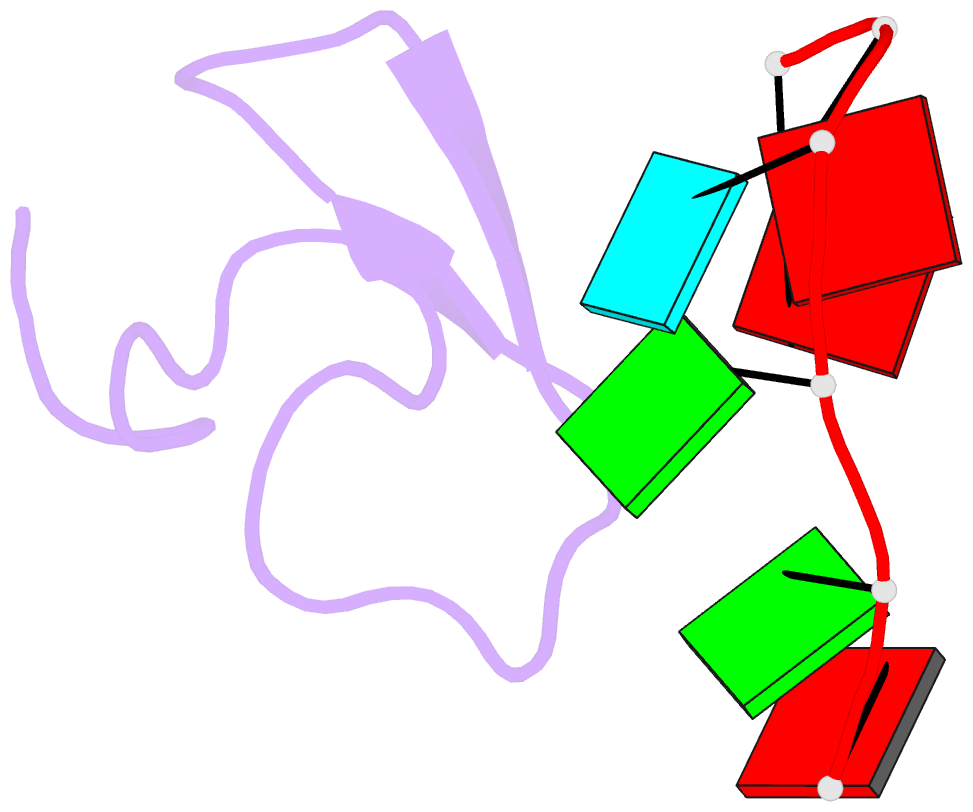
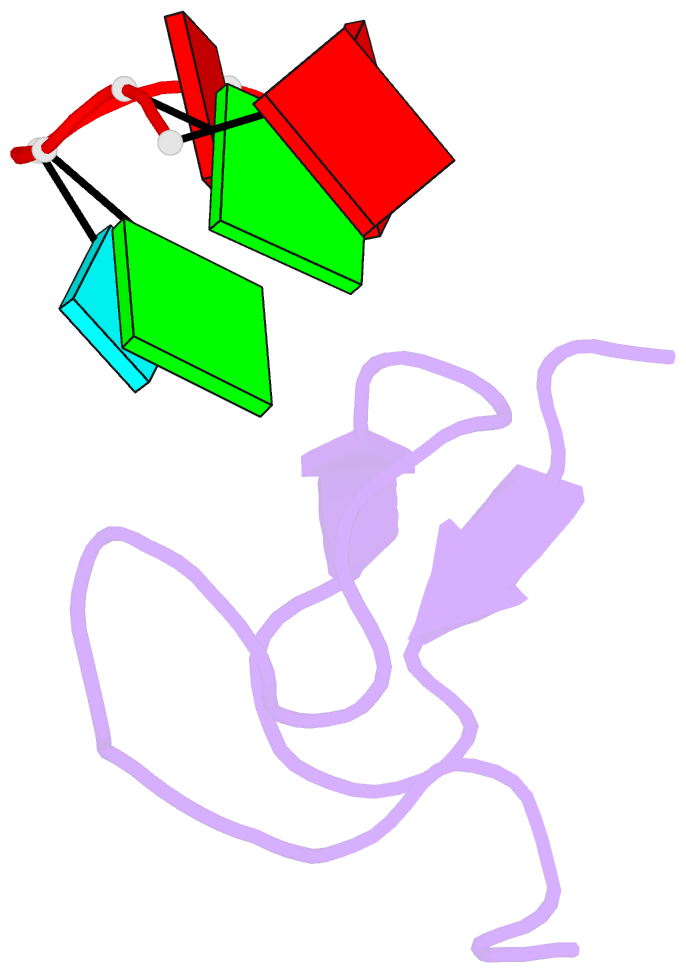
Summary information and primary citation
- PDB-id
- 3g9y; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-RNA
- Method
- X-ray (1.4 Å)
- Summary
- Crystal structure of the second zinc finger from zranb2-znf265 bound to 6 nt ssrna sequence agguaa
- Reference
- Loughlin FE, Mansfield RE, Vaz PM, McGrath AP, Setiyaputra S, Gamsjaeger R, Chen ES, Morris BJ, Guss JM, Mackay JP (2009): "The zinc fingers of the SR-like protein ZRANB2 are single-stranded RNA-binding domains that recognize 5' splice site-like sequences." Proc.Natl.Acad.Sci.USA, 106, 5581-5586. doi: 10.1073/pnas.0802466106.
- Abstract
- The alternative splicing of mRNA is a critical process in higher eukaryotes that generates substantial proteomic diversity. Many of the proteins that are essential to this process contain arginine/serine-rich (RS) domains. ZRANB2 is a widely-expressed and highly-conserved RS-domain protein that can regulate alternative splicing but lacks canonical RNA-binding domains. Instead, it contains 2 RanBP2-type zinc finger (ZnF) domains. We demonstrate that these ZnFs recognize ssRNA with high affinity and specificity. Each ZnF binds to a single AGGUAA motif and the 2 domains combine to recognize AGGUAA(N(x))AGGUAA double sites, suggesting that ZRANB2 regulates alternative splicing via a direct interaction with pre-mRNA at sites that resemble the consensus 5' splice site. We show using X-ray crystallography that recognition of an AGGUAA motif by a single ZnF is dominated by side-chain hydrogen bonds to the bases and formation of a guanine-tryptophan-guanine "ladder." A number of other human proteins that function in RNA processing also contain RanBP2 ZnFs in which the RNA-binding residues of ZRANB2 are conserved. The ZnFs of ZRANB2 therefore define another class of RNA-binding domain, advancing our understanding of RNA recognition and emphasizing the versatility of ZnF domains in molecular recognition.