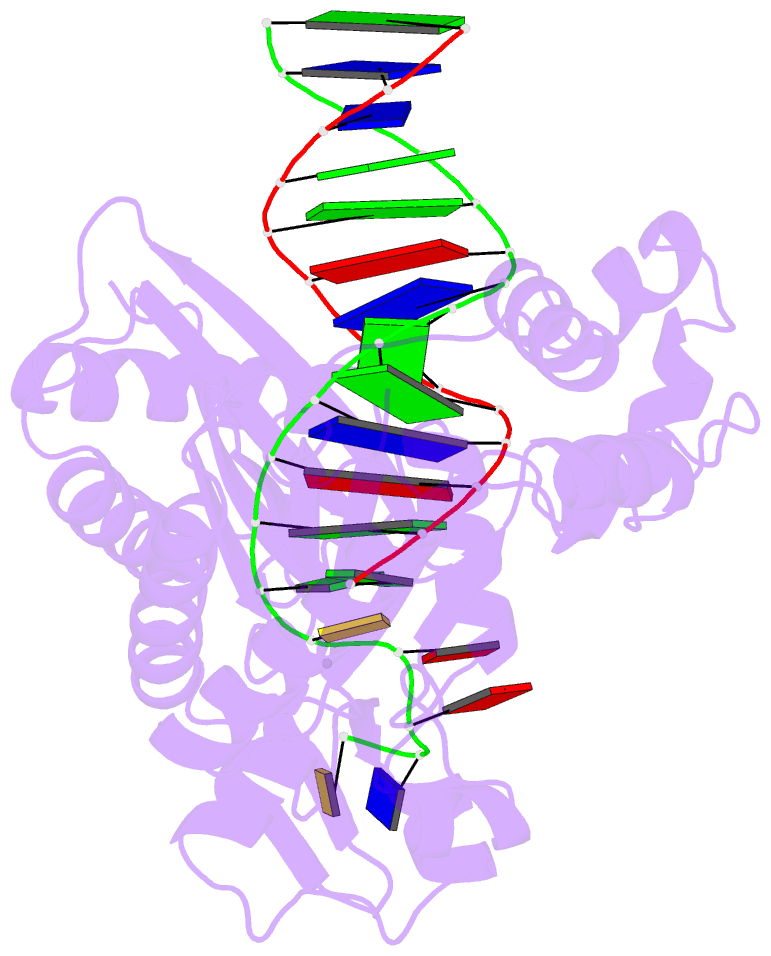
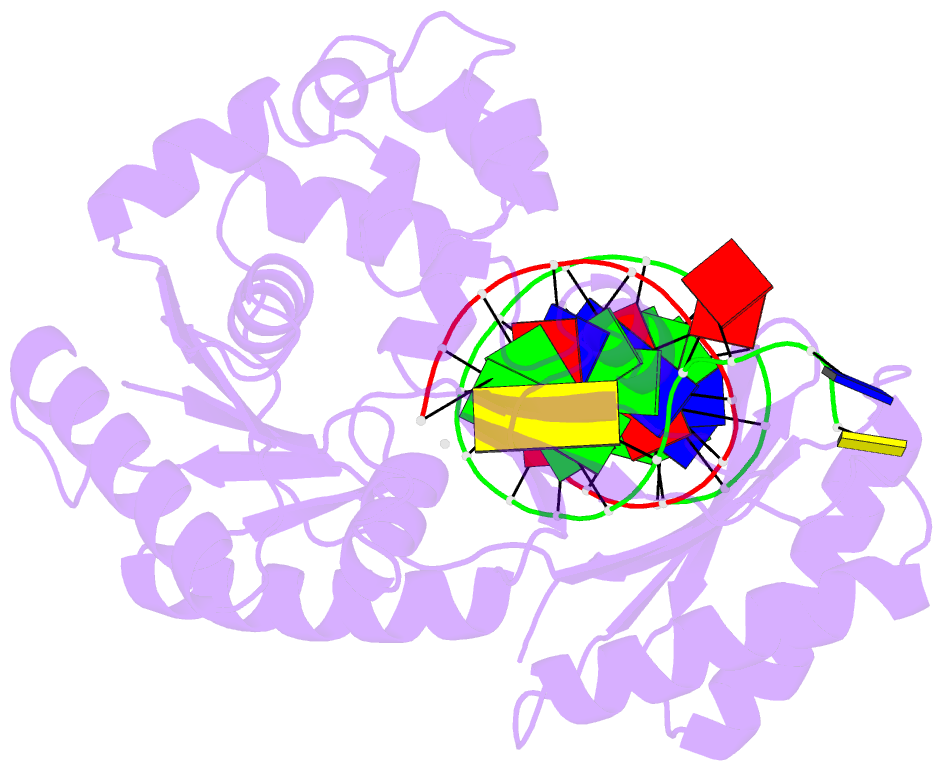
Summary information and primary citation
- PDB-id
- 3gim; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.7 Å)
- Summary
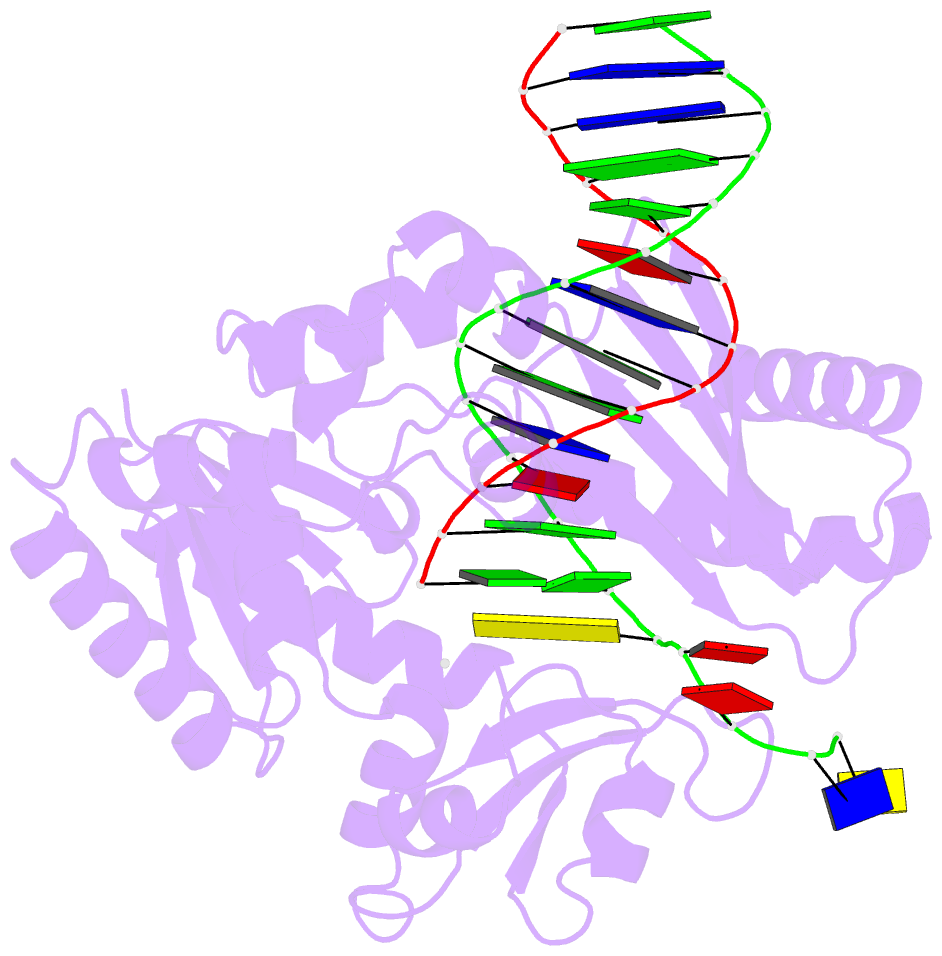
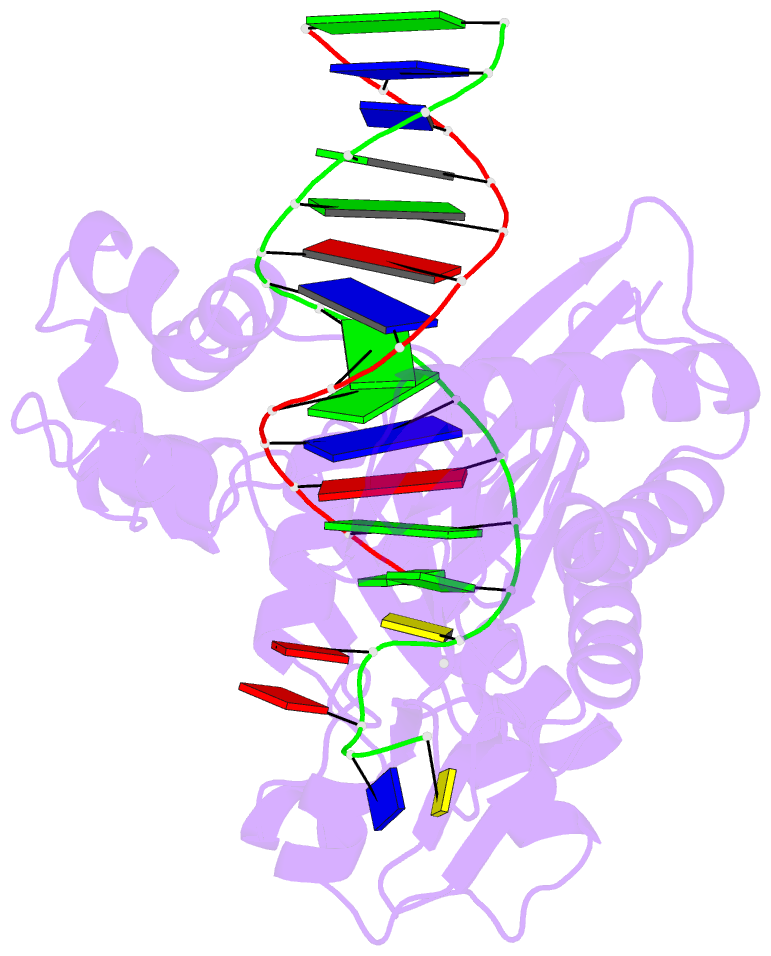
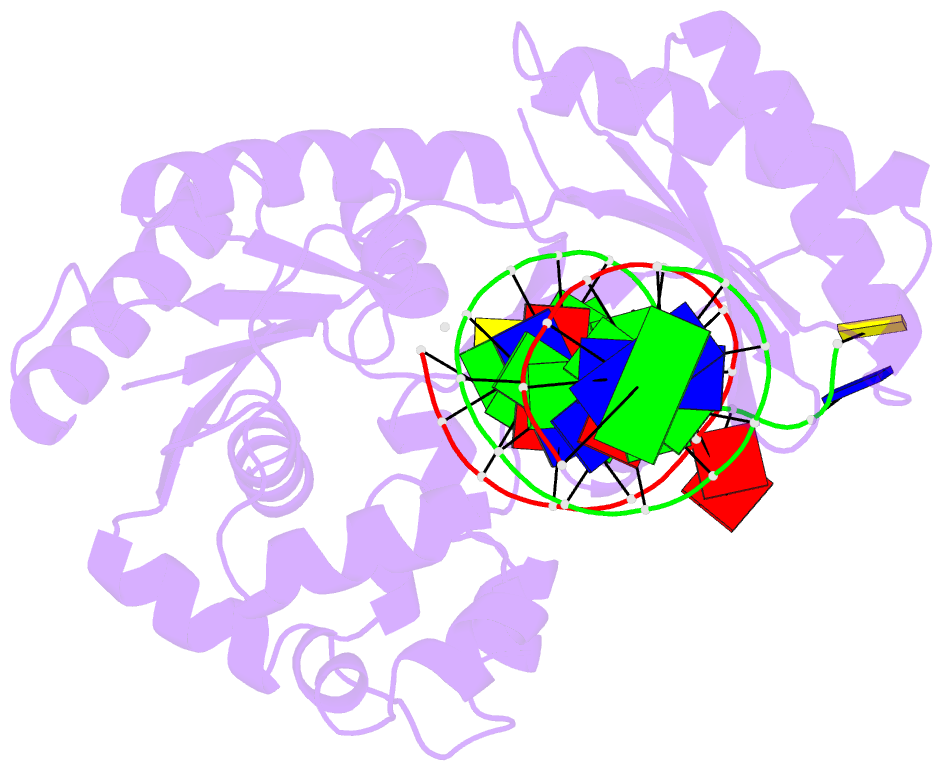
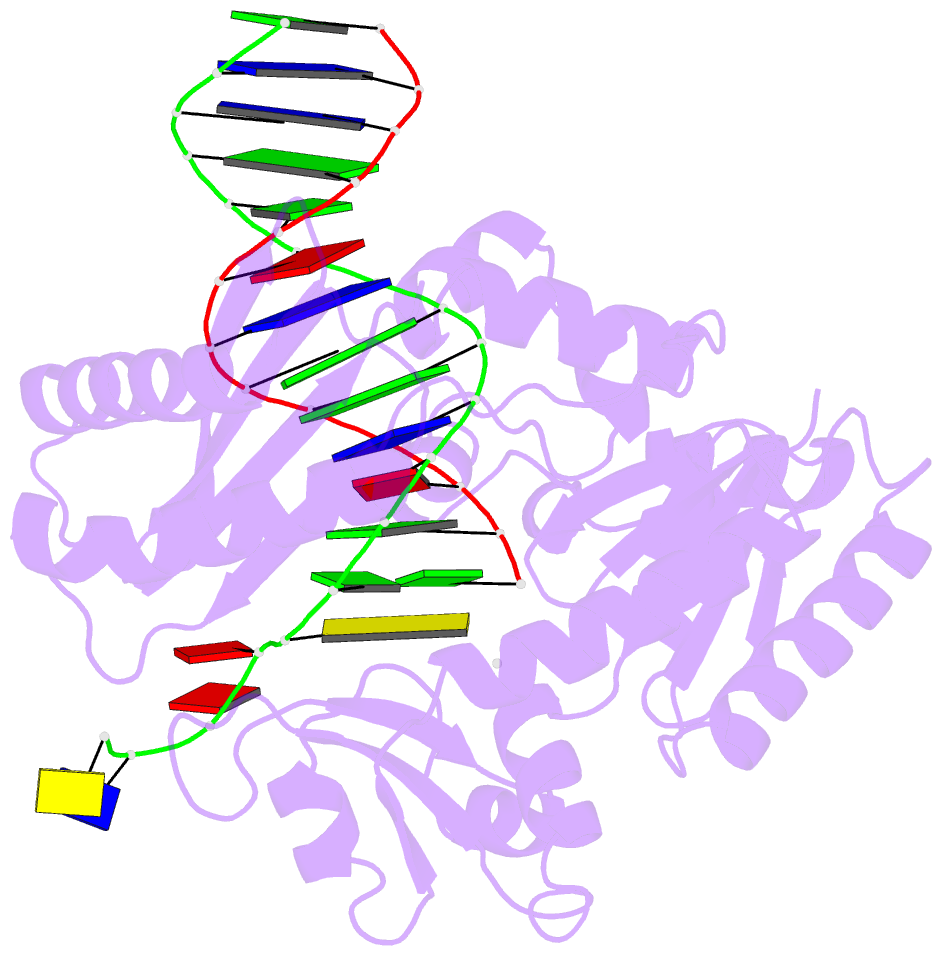
- Dpo4 extension ternary complex with oxog(anti)-g(syn) pair
- Reference
- Rechkoblit O, Malinina L, Cheng Y, Geacintov NE, Broyde S, Patel DJ (2009): "Impact of conformational heterogeneity of OxoG lesions and their pairing partners on bypass fidelity by Y family polymerases." Structure, 17, 725-736. doi: 10.1016/j.str.2009.03.011.
- Abstract
- 7,8-Dihydro-8-oxoguanine (oxoG), the predominant oxidative DNA damage lesion, is processed differently by high-fidelity and Y-family lesion bypass polymerases. Although high-fidelity polymerases extend predominantly from an A base opposite an oxoG, the Y-family polymerases Dpo4 and human Pol eta preferentially extend from the oxoG*C base pair. We have determined crystal structures of extension Dpo4 ternary complexes with oxoG opposite C, A, G, or T and the next nascent base pair. We demonstrate that neither template backbone nor the architecture of the active site is perturbed by the oxoG(anti)*C and oxoG*A pairs. However, the latter manifest conformational heterogeneity, adopting both oxoG(syn)*A(anti) and oxoG(anti)*A(syn) alignment. Hence, the observed reduced primer extension from the dynamically flexible 3'-terminal primer base A is explained. Because of homology between Dpo4 and Pol eta, such a dynamic screening mechanism might be utilized by Dpo4 and Pol eta to regulate error-free versus error-prone bypass of oxoG and other lesions.