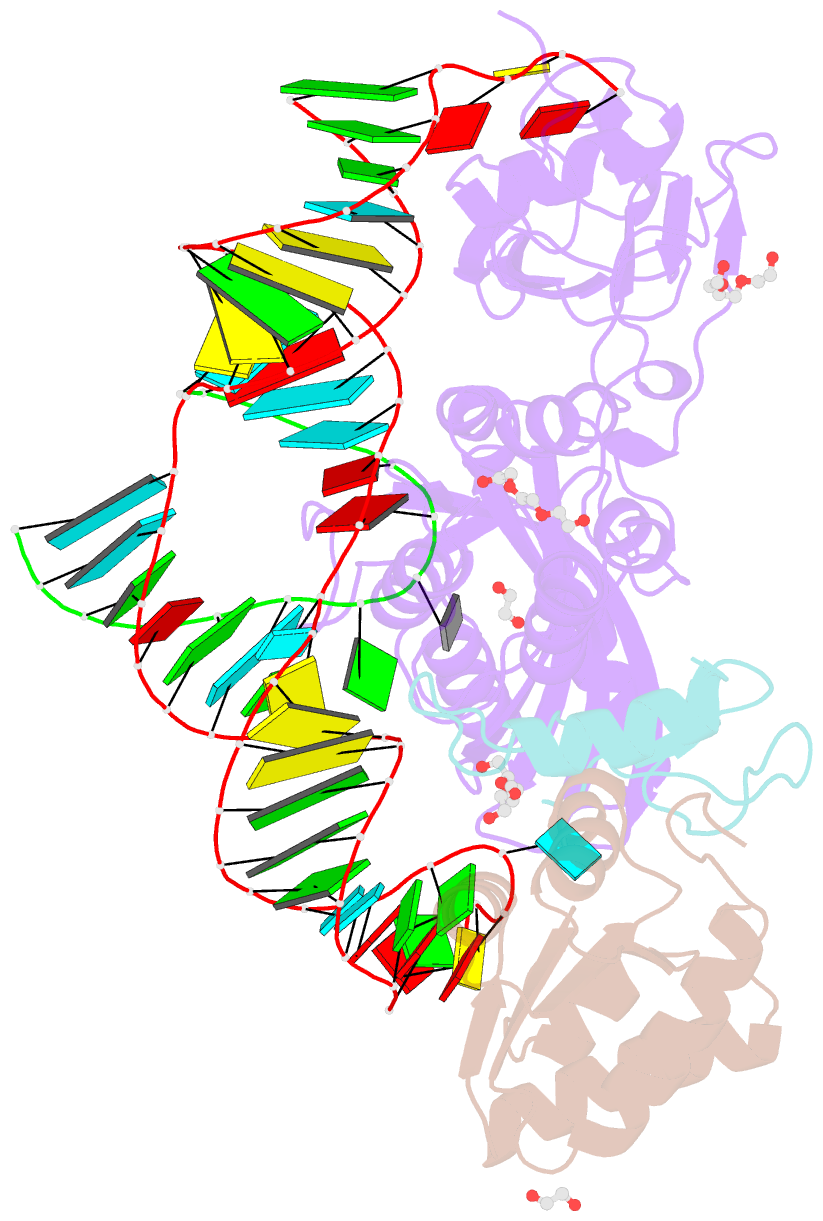
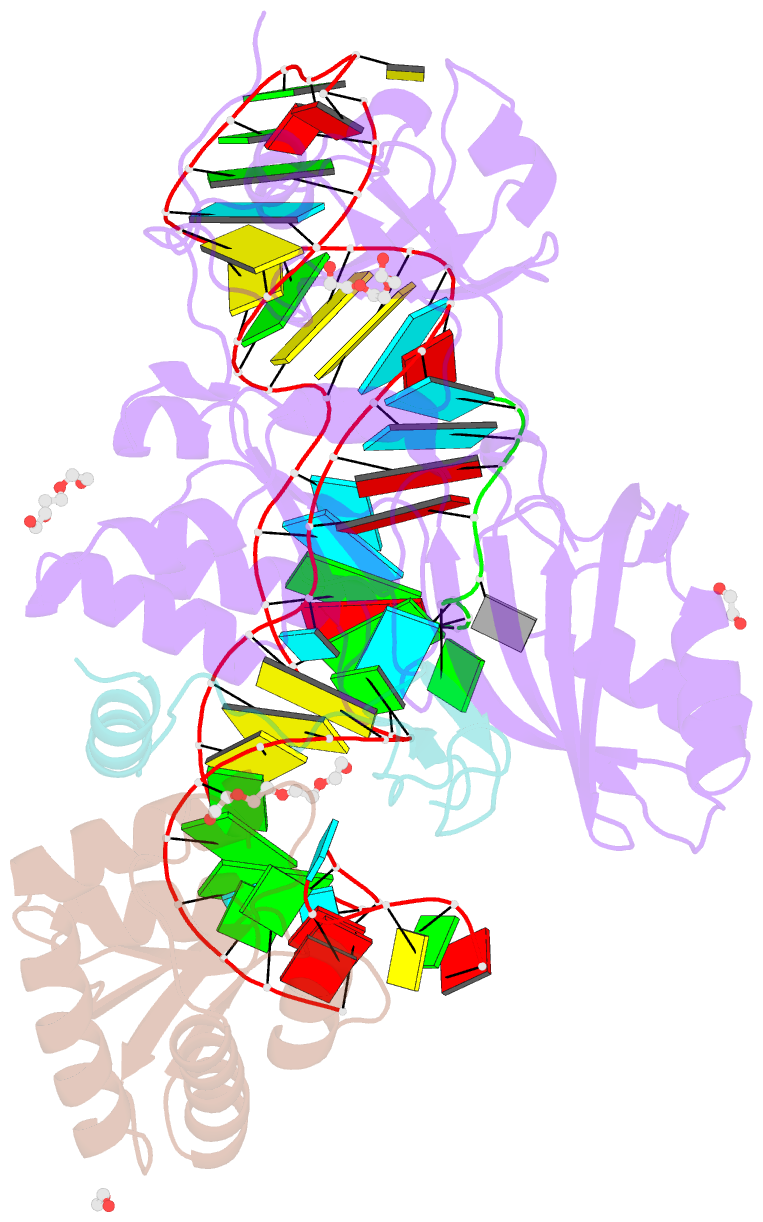
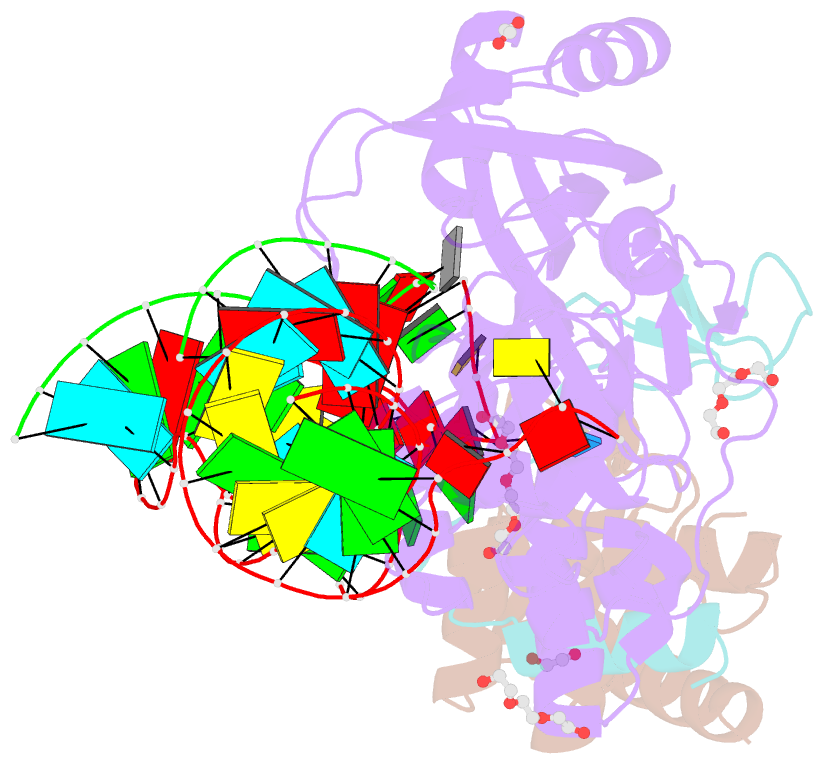
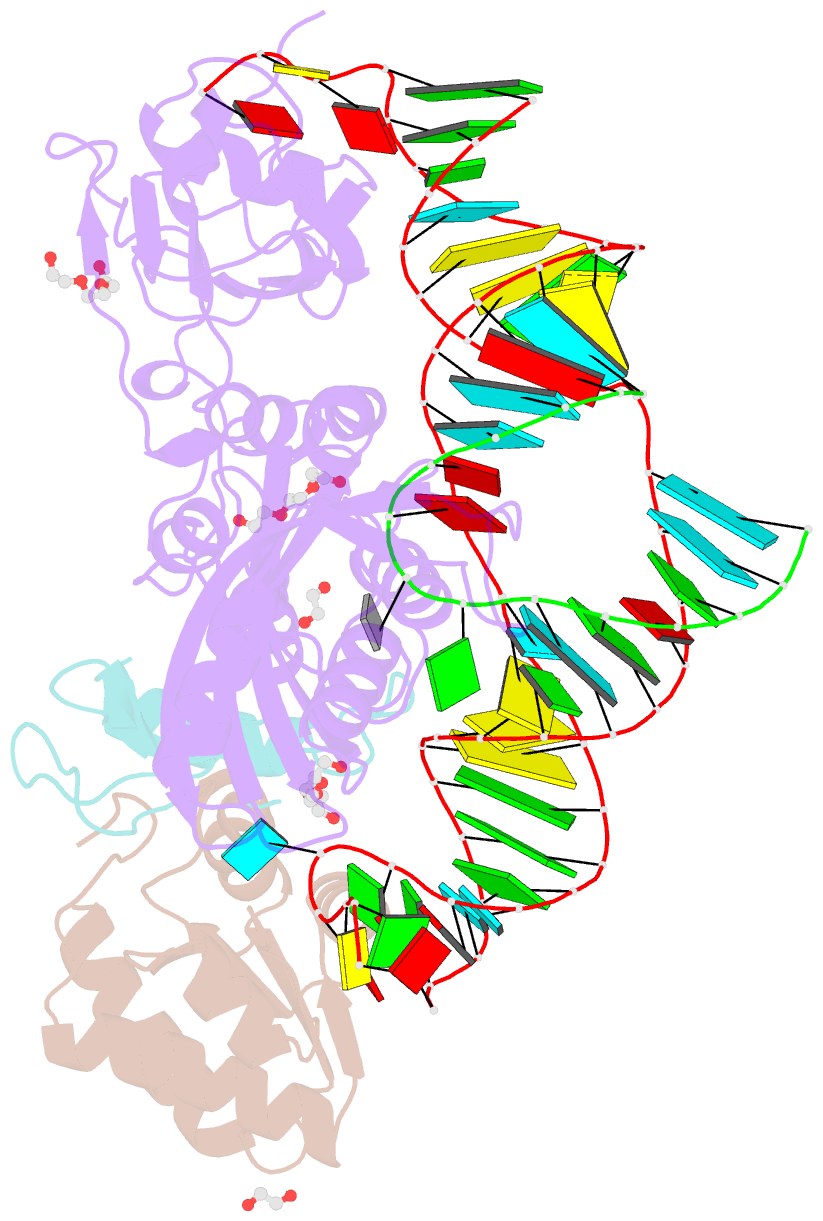
Summary information and primary citation
- PDB-id
- 3hax; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase-biosynthetic protein-RNA
- Method
- X-ray (2.11 Å)
- Summary
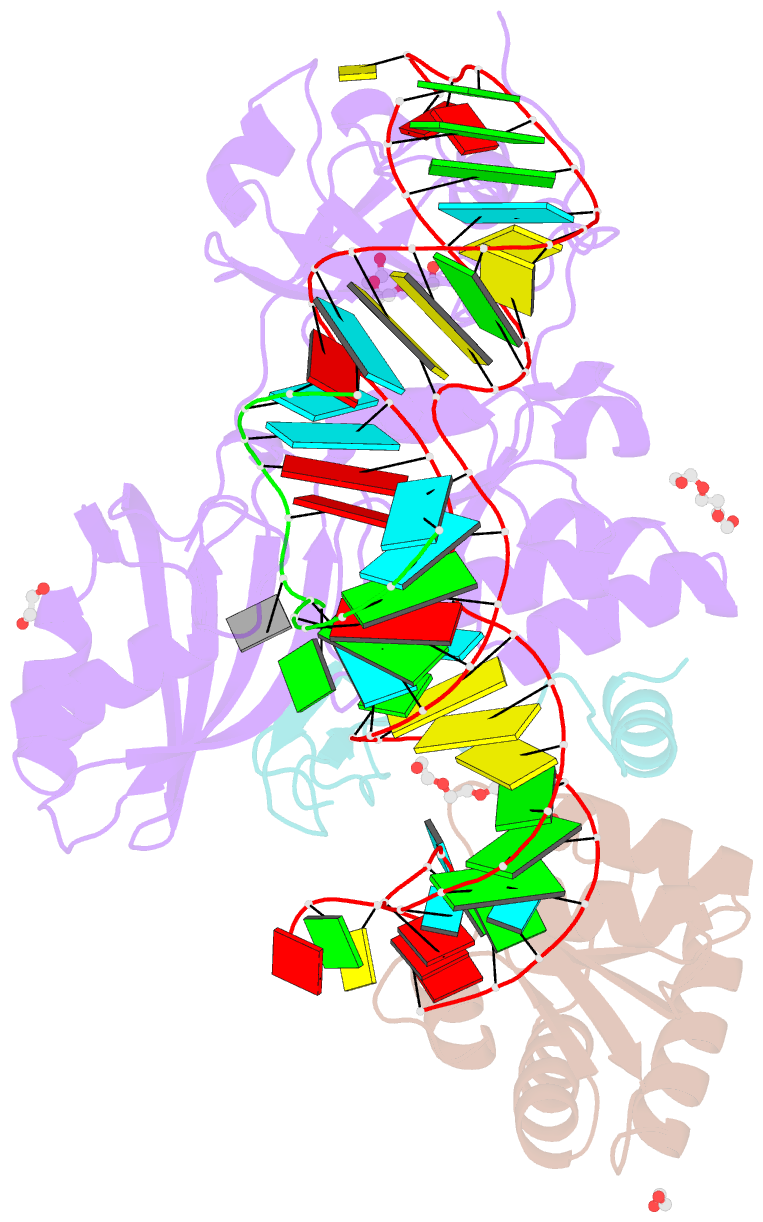
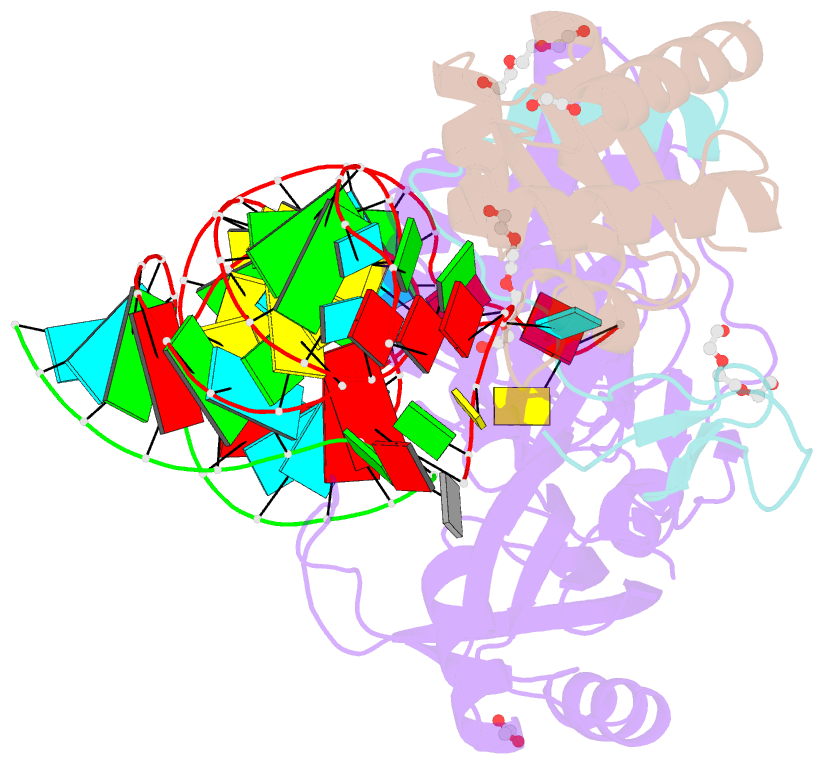
- Crystal structure of a substrate-bound gar1-minus h-aca rnp from pyrococcus furiosus
- Reference
- Duan J, Li L, Lu J, Wang W, Ye K (2009): "Structural mechanism of substrate RNA recruitment in H/ACA RNA-guided pseudouridine synthase." Mol.Cell, 34, 427-439. doi: 10.1016/j.molcel.2009.05.005.
- Abstract
- H/ACA RNAs form ribonucleoprotein complex (RNP) with proteins Cbf5, Nop10, L7Ae, and Gar1 and guide site-specific conversion of uridine into pseudouridine in cellular RNAs. The crystal structures of H/ACA RNP with substrate bound at the active site cleft reveal that the substrate is recruited through sequence-specific pairing with guide RNA and essential protein contacts. Substrate binding leads to a reorganization of a preset pseudouridylation pocket and an adaptive movement of the PUA domain and the lower stem of the H/ACA RNA. Moreover, a thumb loop flips from the Gar1-bound state in the substrate-free RNP structure to tightly associate with the substrate. Mutagenesis and enzyme kinetics analysis suggest a critical role of Gar1 and the thumb in substrate turnover, particularly in product release. Comparison with tRNA Psi55 synthase TruB reveals the structural conservation and adaptation between an RNA-guided and stand-alone pseudouridine synthase and provides insight into the guide-independent activity of Cbf5.