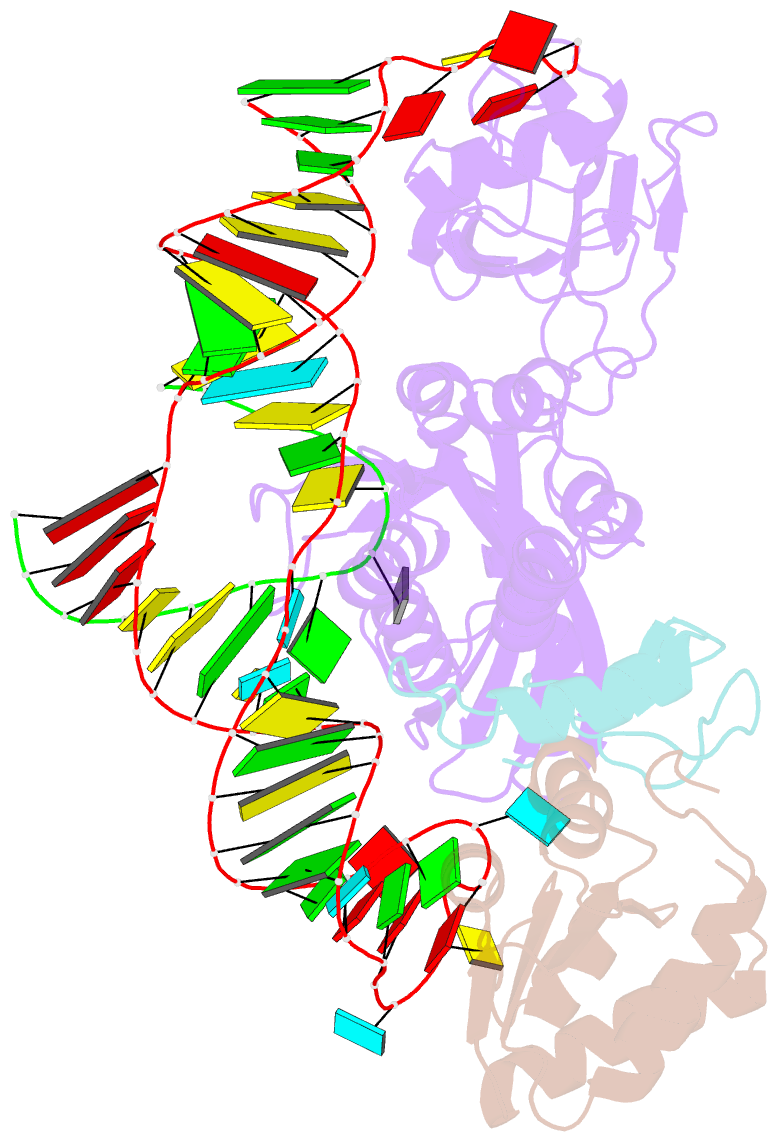
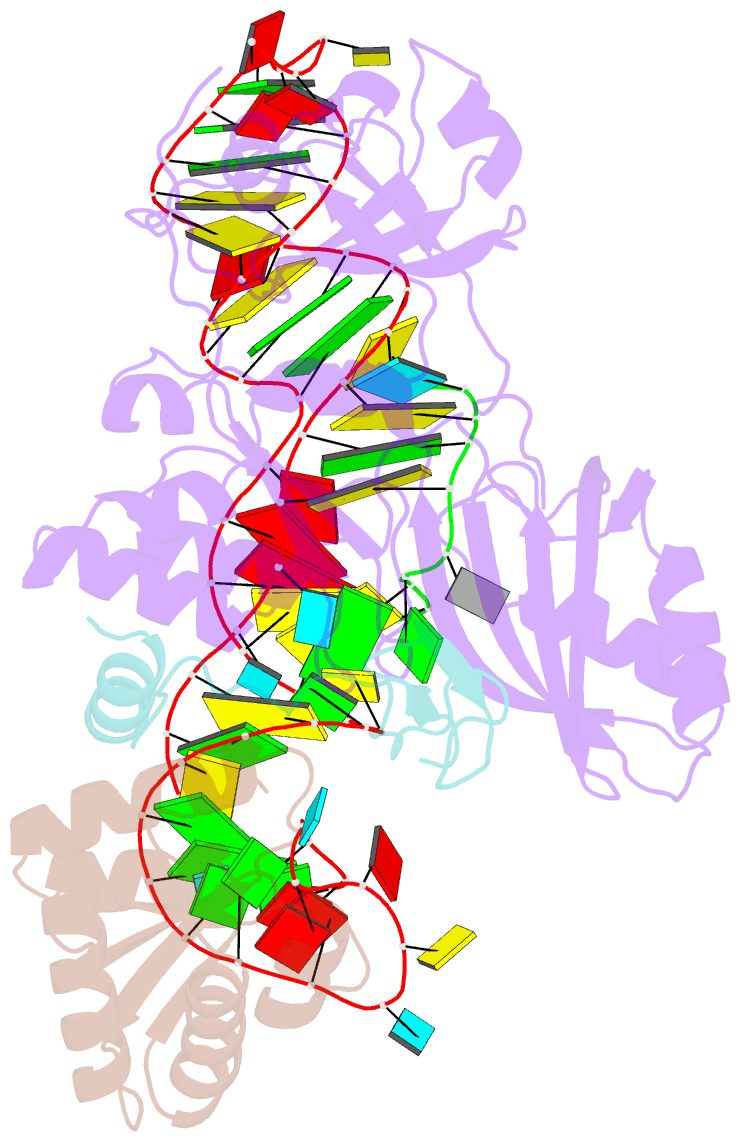
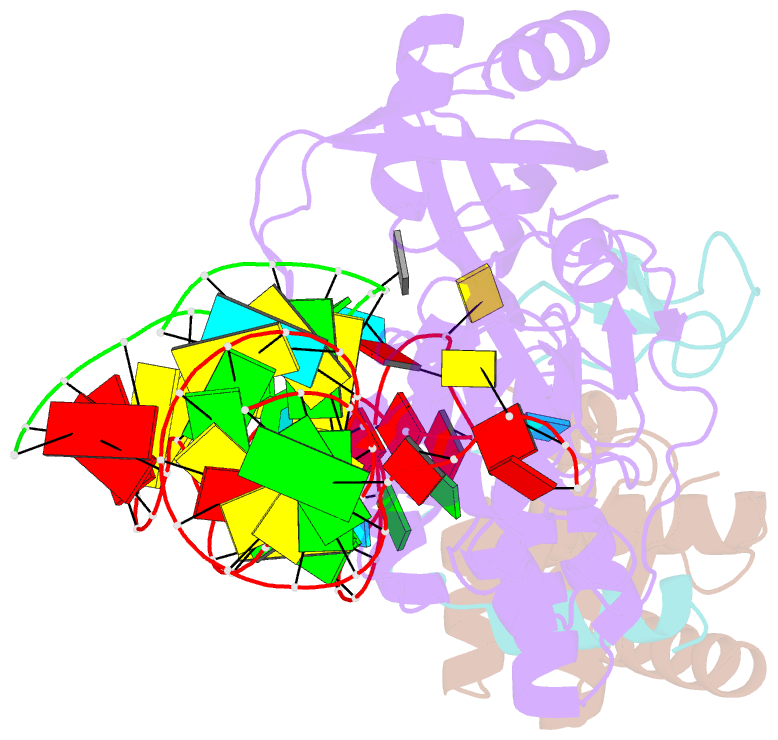
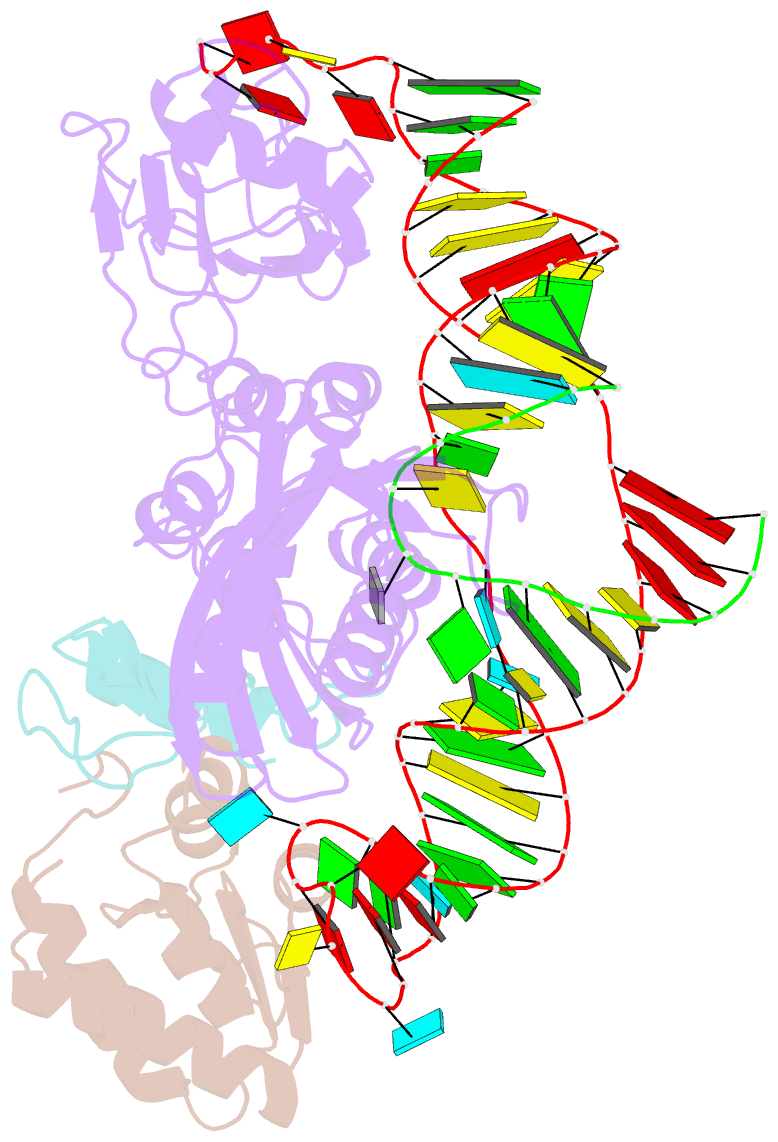
Summary information and primary citation
- PDB-id
- 3hjw; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase-RNA
- Method
- X-ray (2.35 Å)
- Summary
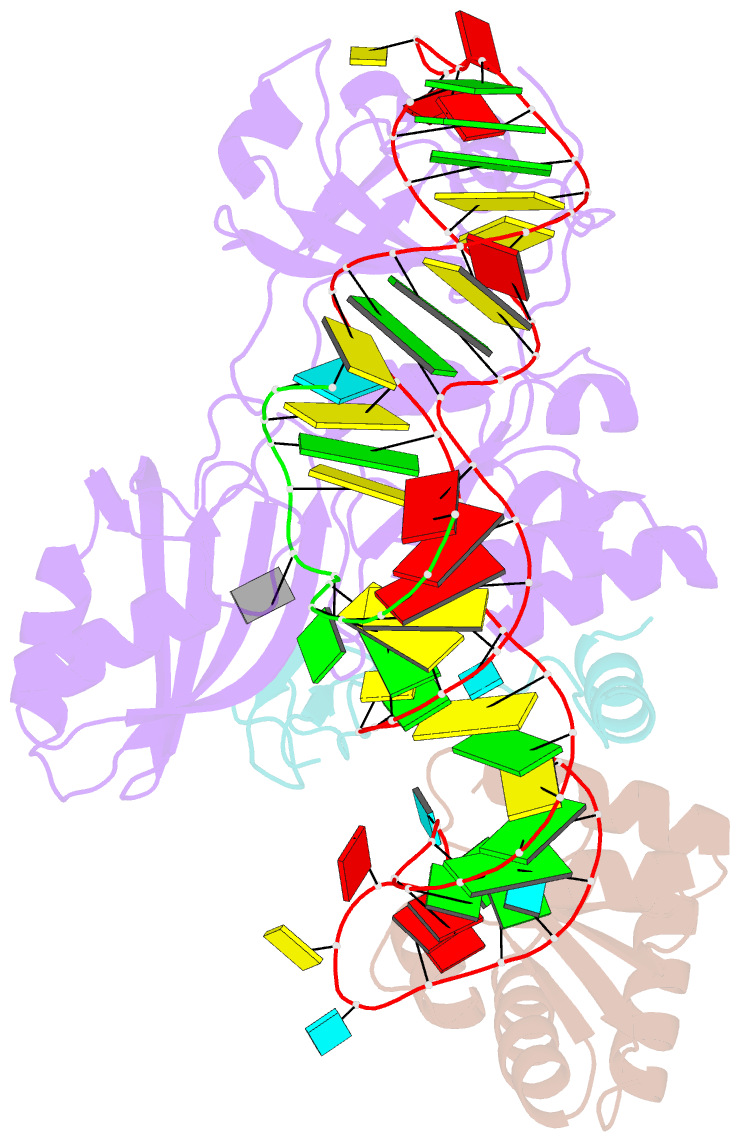
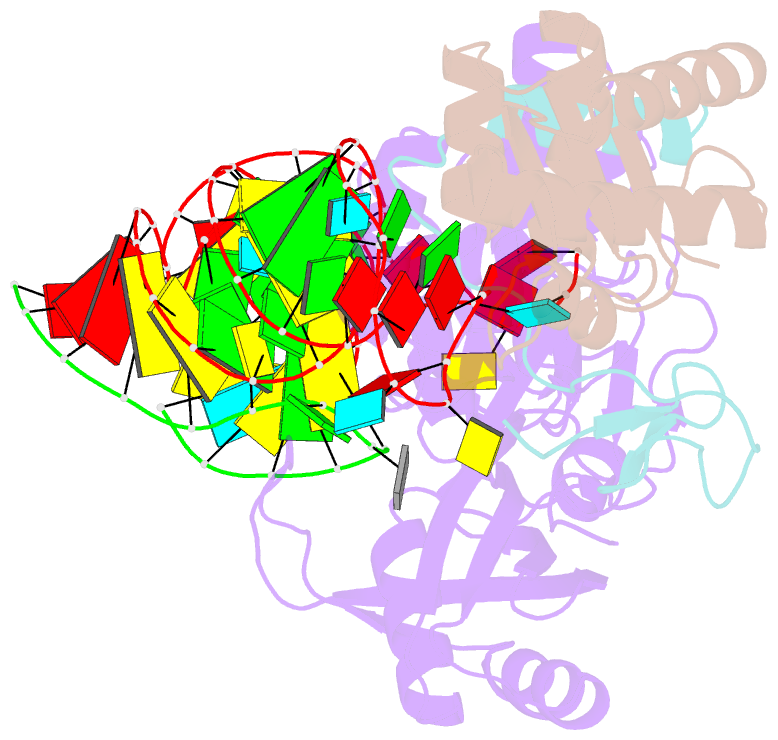
- Structure of a functional ribonucleoprotein pseudouridine synthase bound to a substrate RNA
- Reference
- Liang B, Zhou J, Kahen E, Terns RM, Terns MP, Li H (2009): "Structure of a functional ribonucleoprotein pseudouridine synthase bound to a substrate RNA." Nat.Struct.Mol.Biol., 16, 740-746. doi: 10.1038/nsmb.1624.
- Abstract
- Box H/ACA small nucleolar and Cajal body ribonucleoprotein particles comprise the most complex pseudouridine synthases and are essential for ribosome and spliceosome maturation. The multistep and multicomponent-mediated enzyme mechanism remains only partially understood. Here we report a crystal structure at 2.35 A of a substrate-bound functional archaeal enzyme containing three of the four proteins, Cbf5, Nop10 and L7Ae, and a box H/ACA RNA that reveals detailed information about the protein-only active site. The substrate RNA, containing 5-fluorouridine at the modification position, is fully docked and catalytically rearranged by the enzyme in a manner similar to that seen in two stand-alone pseudouridine synthases. Structural analysis provides a mechanism for plasticity in the diversity of guide RNA sequences used and identifies a substrate-anchoring loop of Cbf5 that also interacts with Gar1 in unliganded structures. Activity analyses of mutated proteins and RNAs support the structural findings and further suggest a role of the Cbf5 loop in regulation of enzyme activity.