Summary information and primary citation
- PDB-id
- 3hts; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (1.75 Å)
- Summary
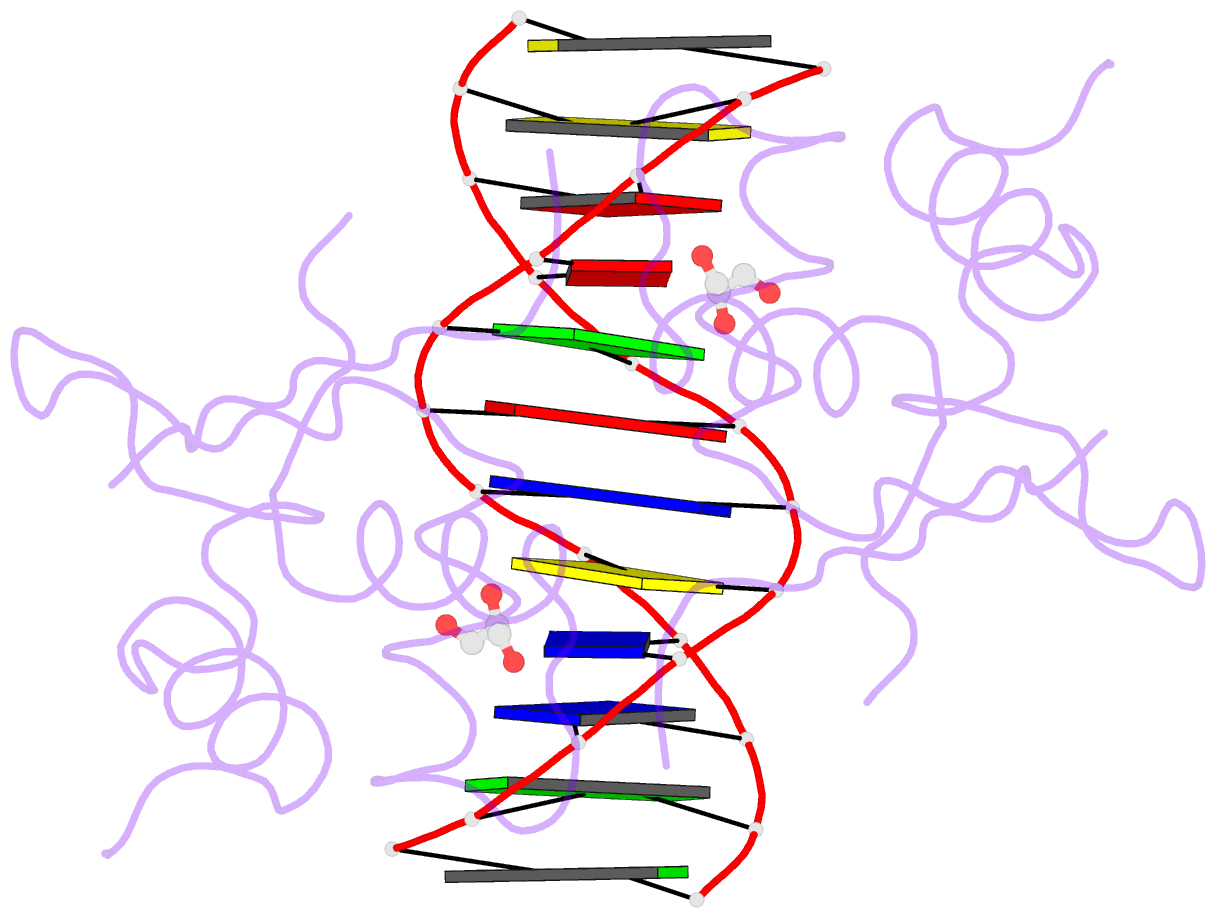
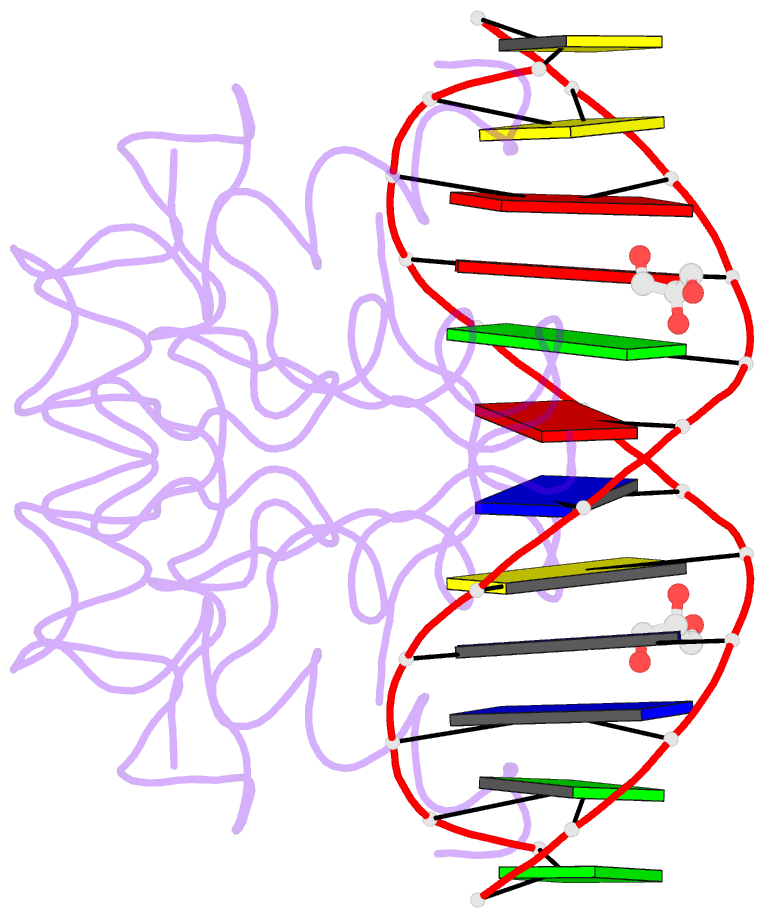
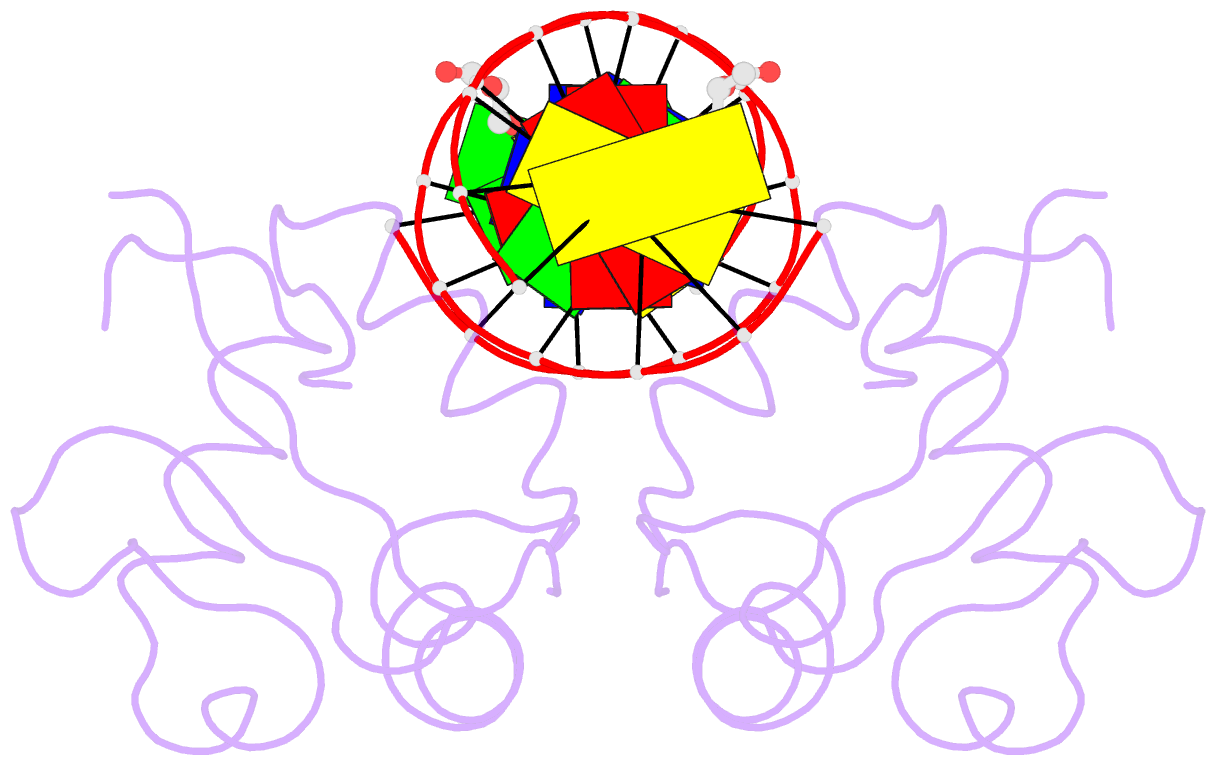
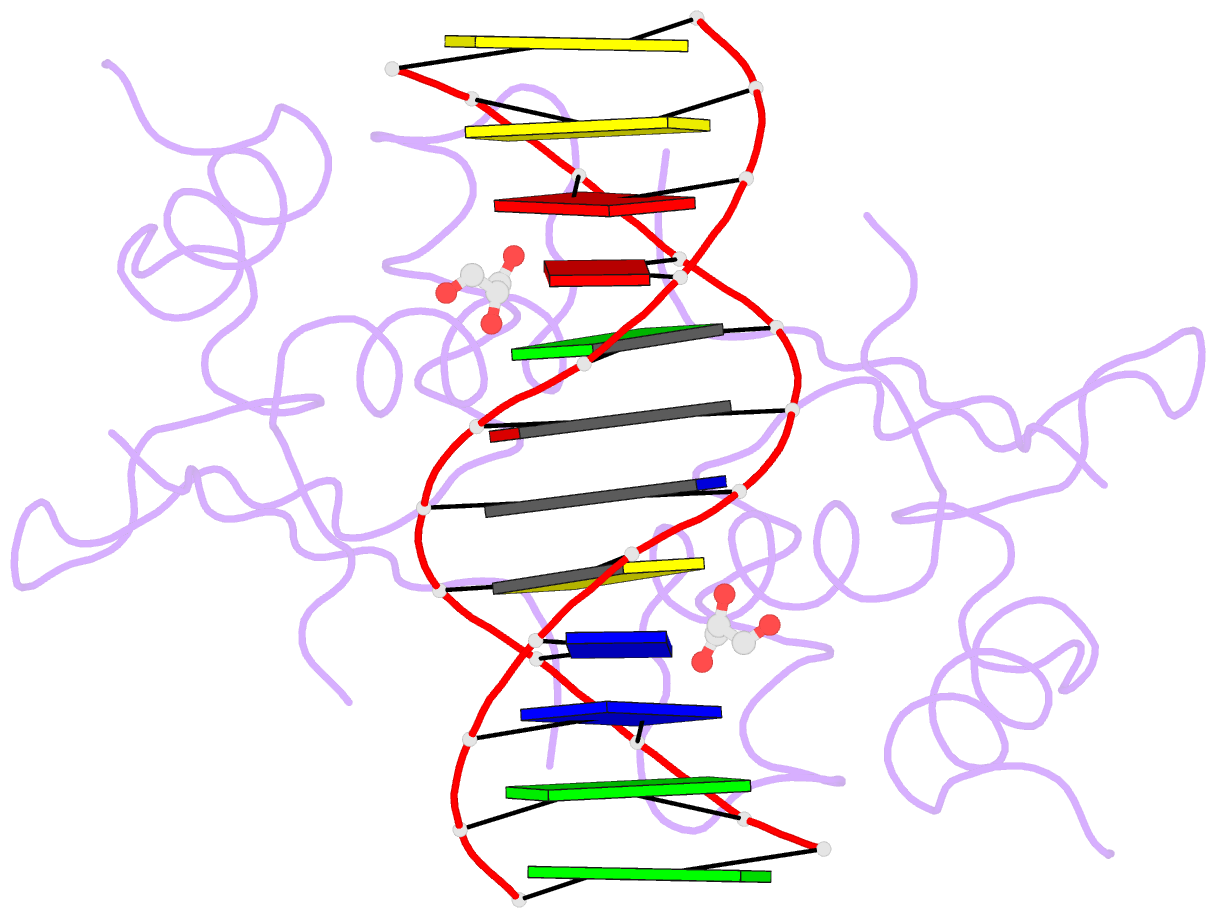
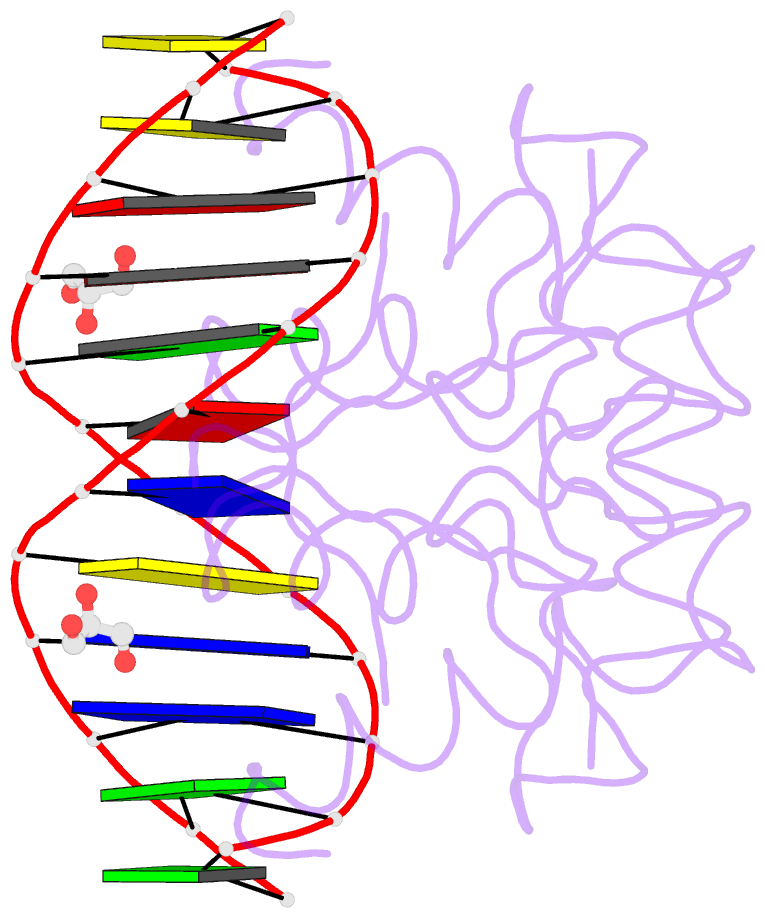
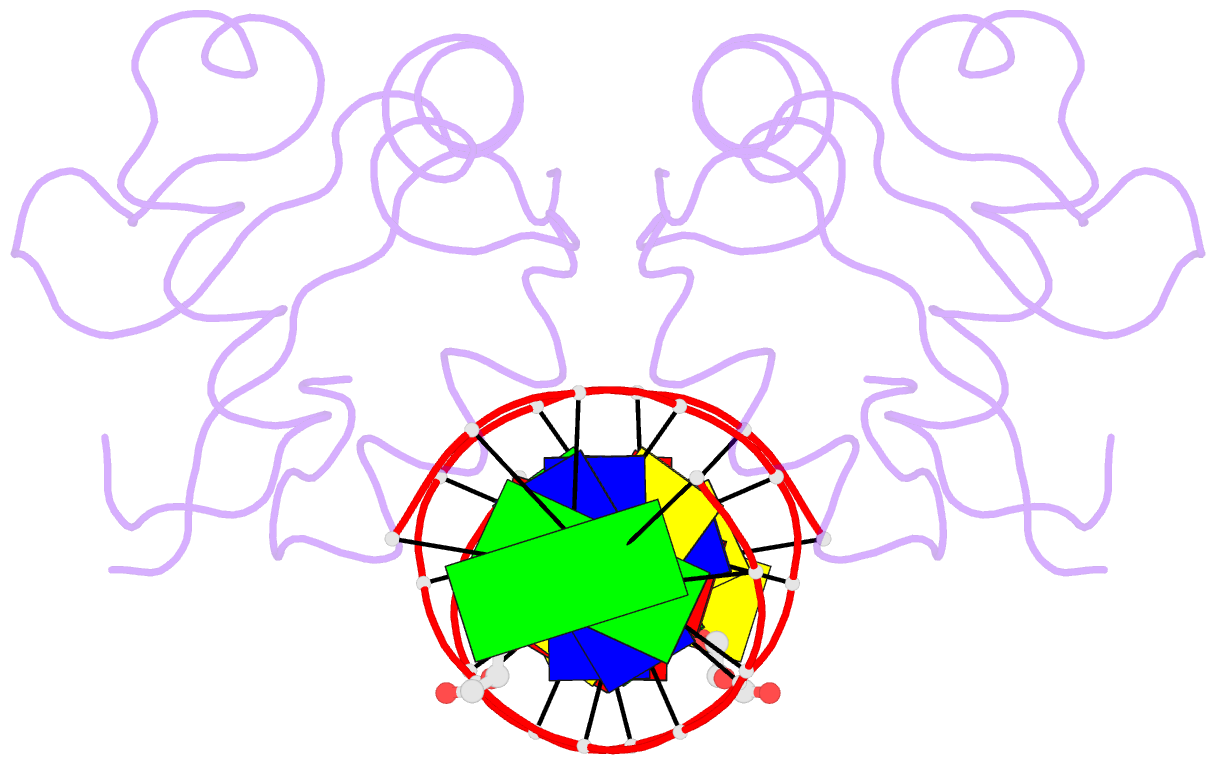
- Heat shock transcription factor-DNA complex
- Reference
- Littlefield O, Nelson HC (1999): "A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF-DNA cocrystal." Nat.Struct.Biol., 6, 464-470. doi: 10.1038/8269.
- Abstract
- The 1.75 A crystal structure of the Kluyveromyces lactis heat shock transcription factor (HSF) DNA-binding domain (DBD) complexed with DNA reveals a protein-DNA interface with few direct major groove contacts and a number of phosphate backbone contacts that are primarily water-mediated interactions. The DBD, a 'winged' helix-turn-helix protein, displays a novel mode of binding in that the 'wing' does not contact DNA like all others of that class. Instead, the monomeric DBD, which crystallized as a symmetric dimer to a pair of nGAAn inverted repeats, uses the 'wing' to form part of the protein-protein contacts. This dimer interface is likely important for increasing the DNA-binding specificity and affinity of the trimeric form of HSF, as well as for increasing cooperativity between adjacent trimers.