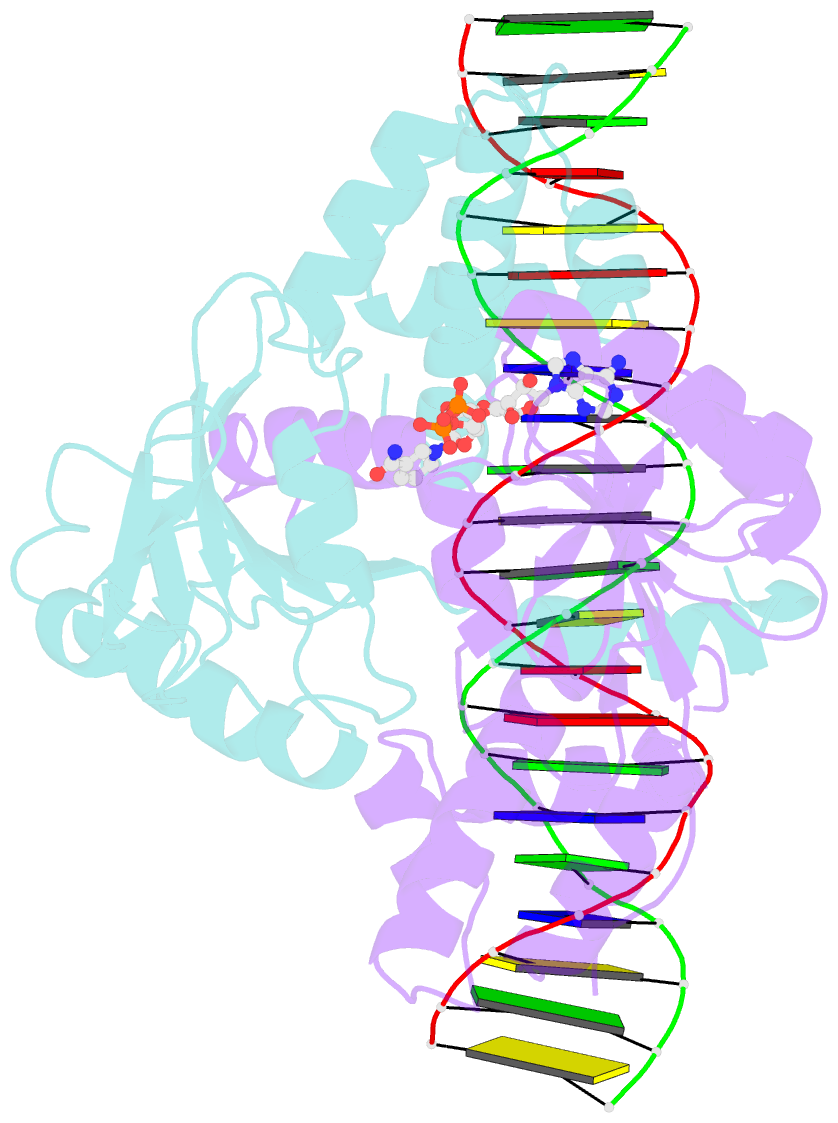
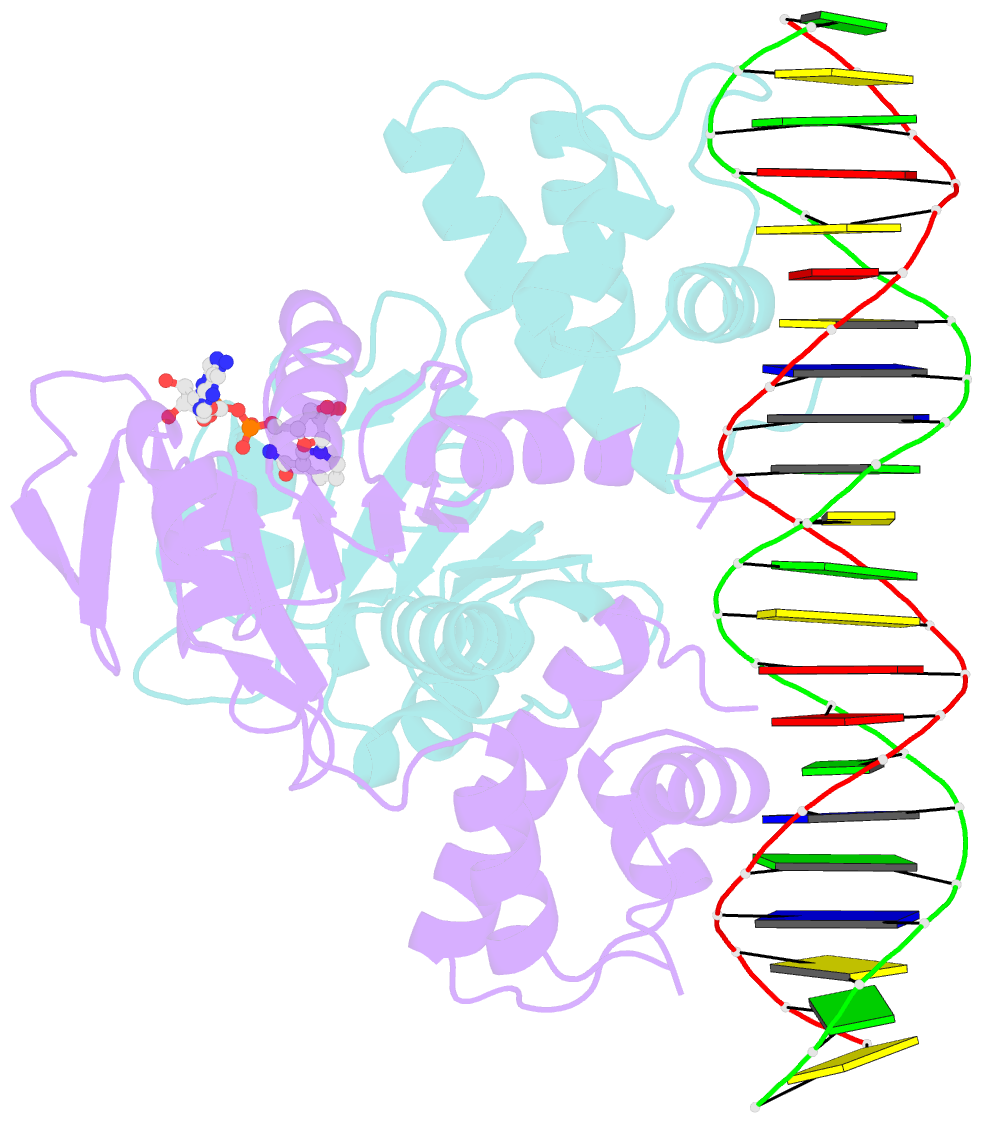
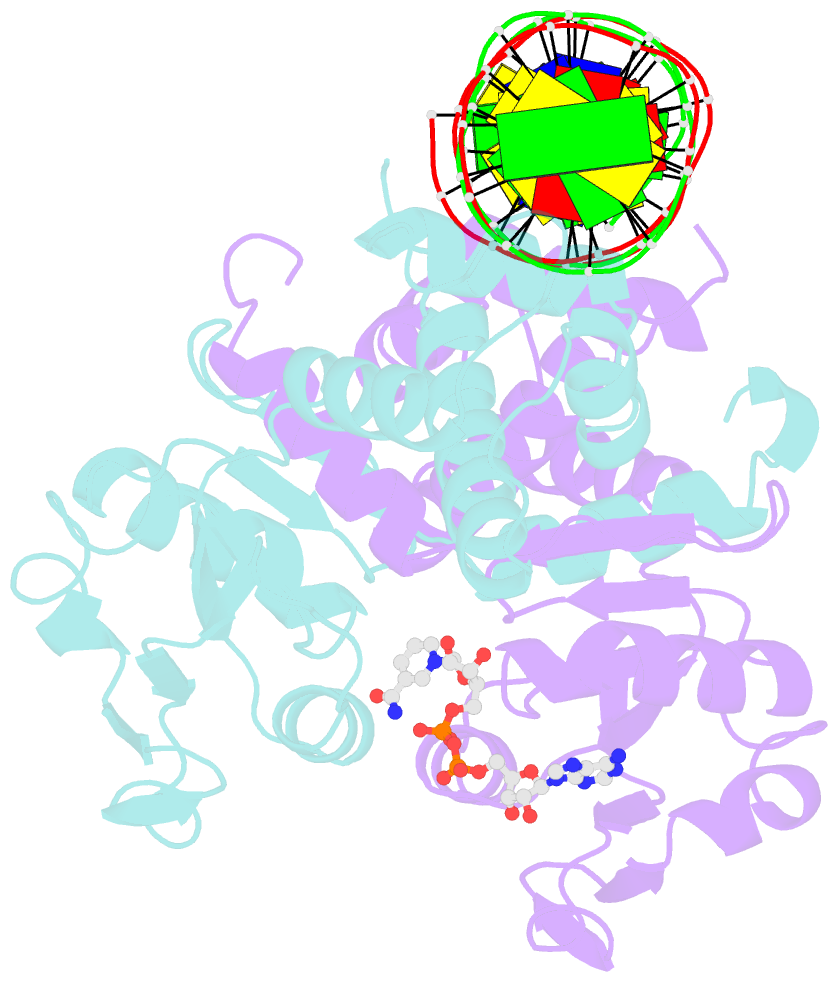
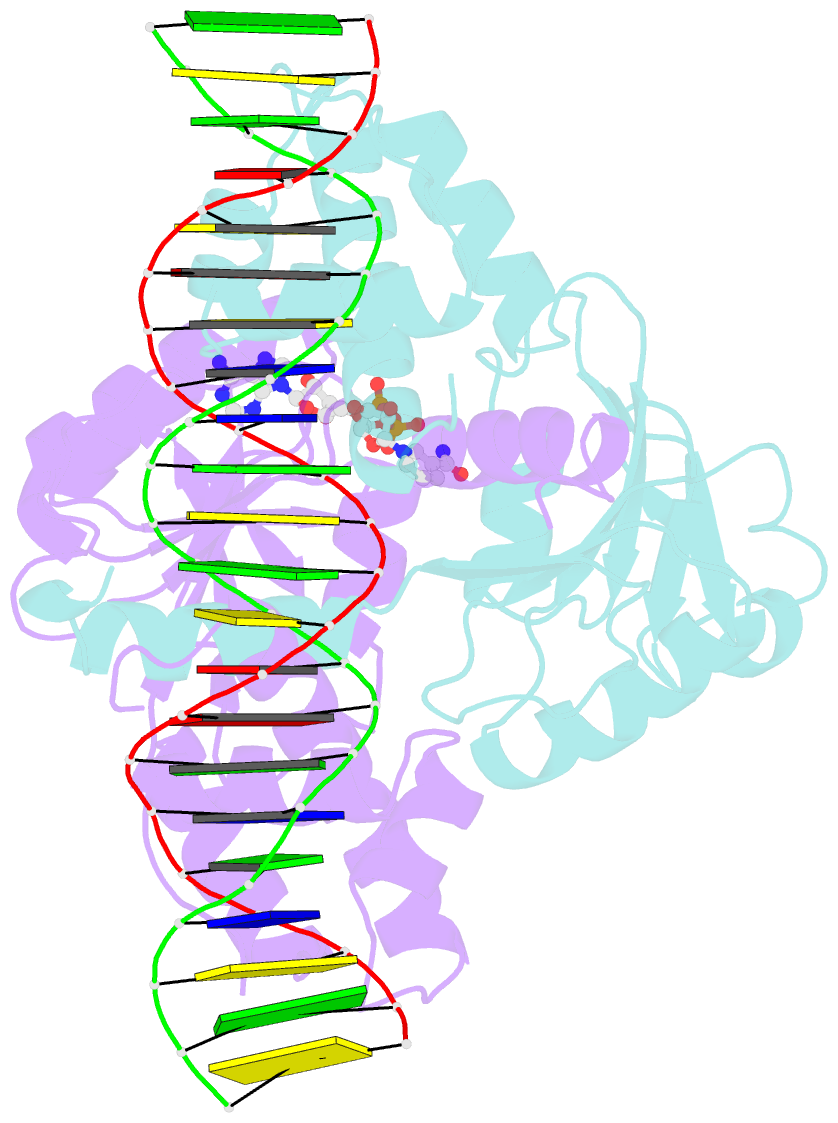
Summary information and primary citation
- PDB-id
- 3ikt; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
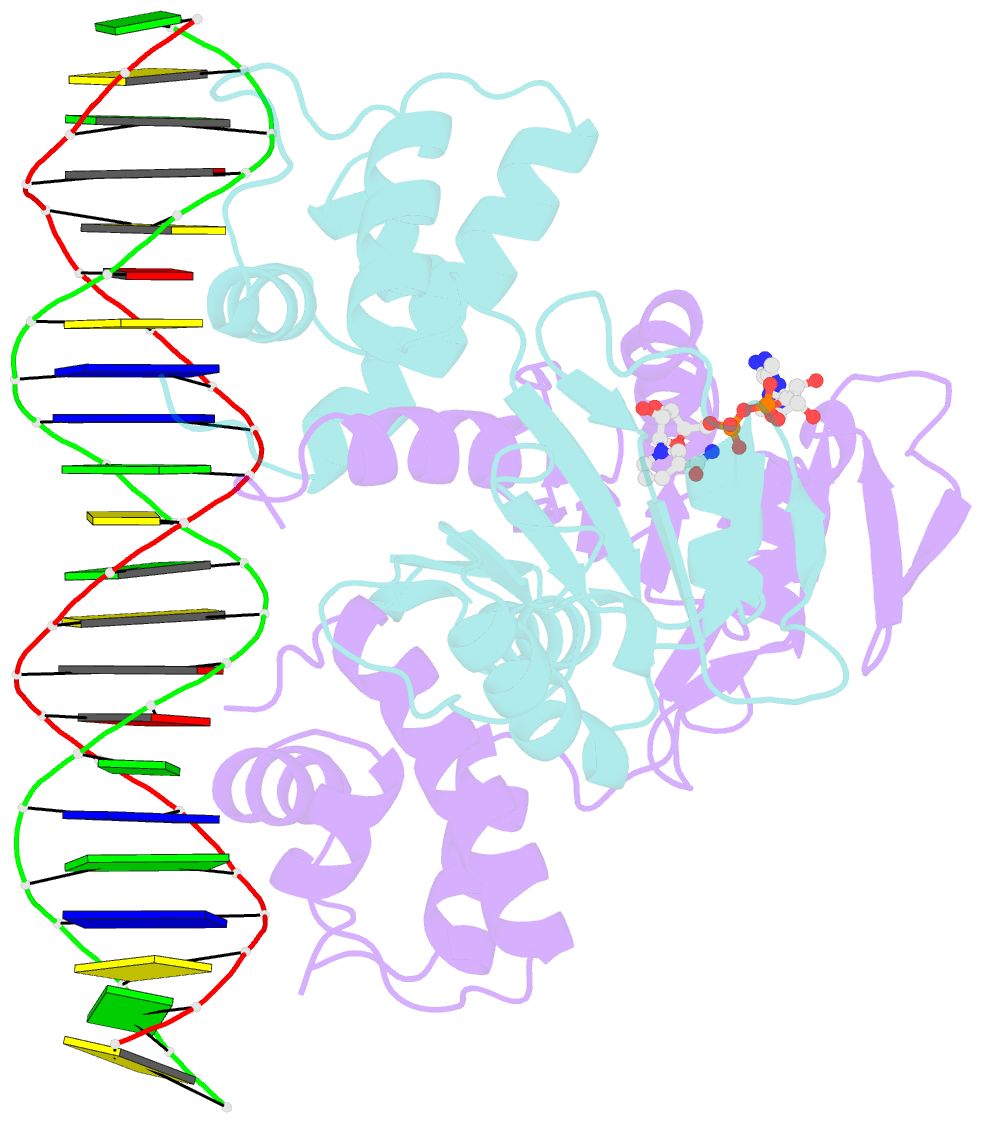
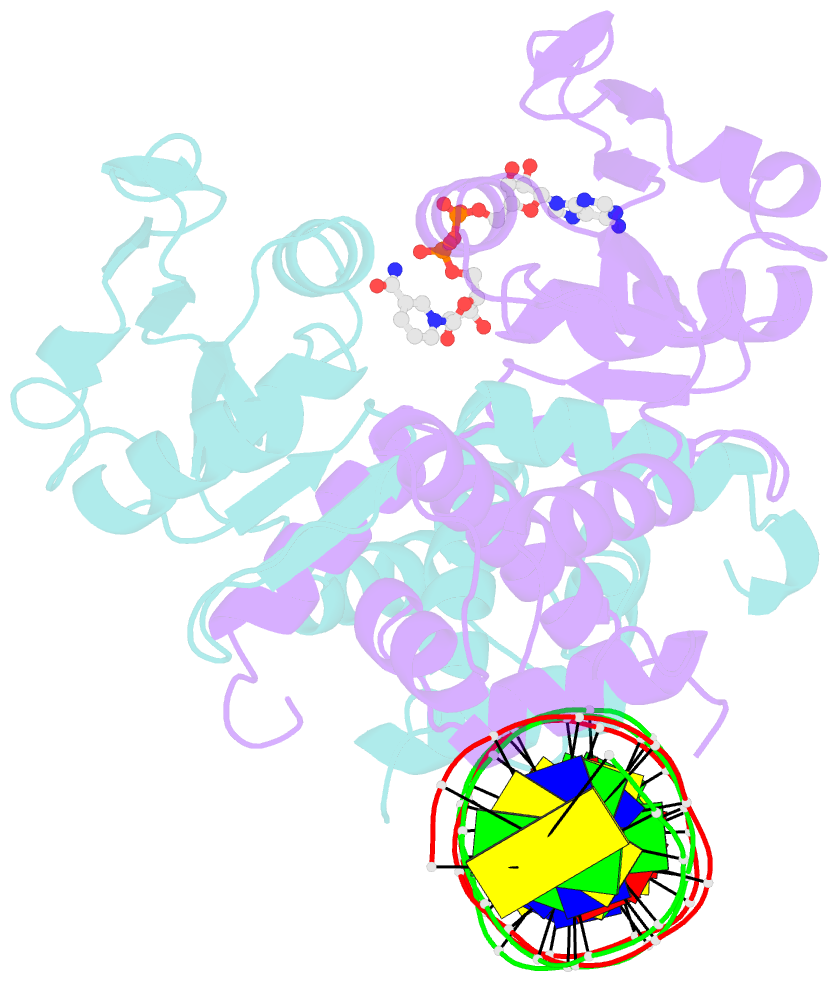
- DNA binding protein-DNA
- Method
- X-ray (2.26 Å)
- Summary
- Crystal structure of a rex-family repressor-DNA-nad+ complex from thermus aquaticus
- Reference
- McLaughlin KJ, Strain-Damerell CM, Xie K, Brekasis D, Soares AS, Paget MS, Kielkopf CL (2010): "Structural basis for NADH/NAD+ redox sensing by a Rex family repressor." Mol.Cell, 38, 563-575. doi: 10.1016/j.molcel.2010.05.006.
- Abstract
- Nicotinamide adenine dinucleotides have emerged as key signals of the cellular redox state. Yet the structural basis for allosteric gene regulation by the ratio of reduced NADH to oxidized NAD(+) is poorly understood. A key sensor among Gram-positive bacteria, Rex represses alternative respiratory gene expression until a limited oxygen supply elevates the intracellular NADH:NAD(+) ratio. Here we investigate the molecular mechanism for NADH/NAD(+) sensing among Rex family members by determining structures of Thermus aquaticus Rex bound to (1) NAD(+), (2) DNA operator, and (3) without ligand. Comparison with the Rex/NADH complex reveals that NADH releases Rex from the DNA site following a 40 degrees closure between the dimeric subunits. Complementary site-directed mutagenesis experiments implicate highly conserved residues in NAD-responsive DNA-binding activity. These rare views of a redox sensor in action establish a means for slight differences in the nicotinamide charge, pucker, and orientation to signal the redox state of the cell.