Summary information and primary citation
- PDB-id
- 3j3w; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (10.7 Å)
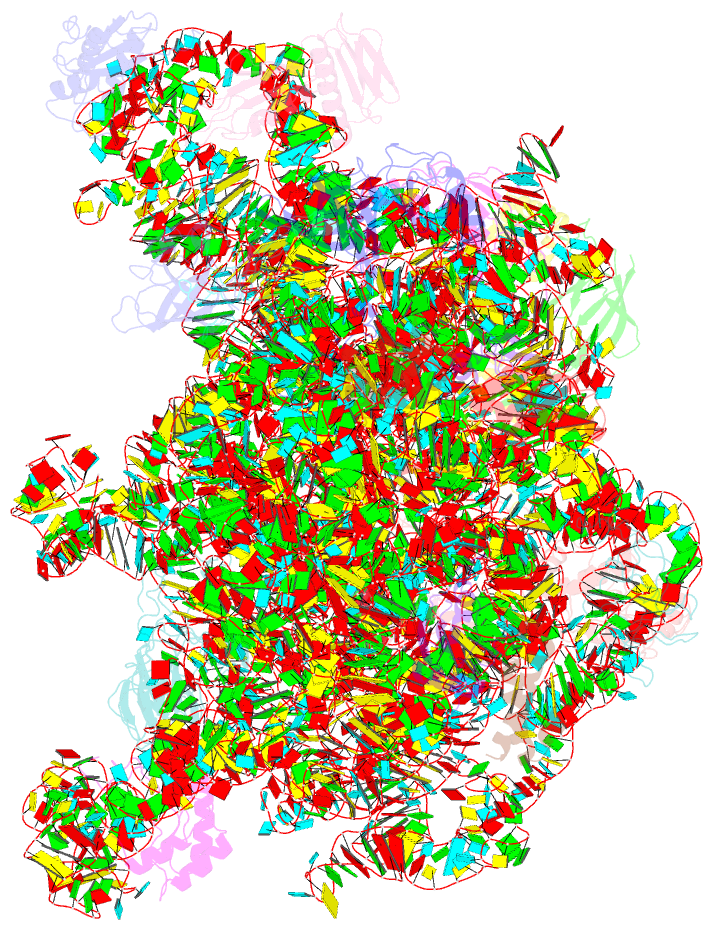
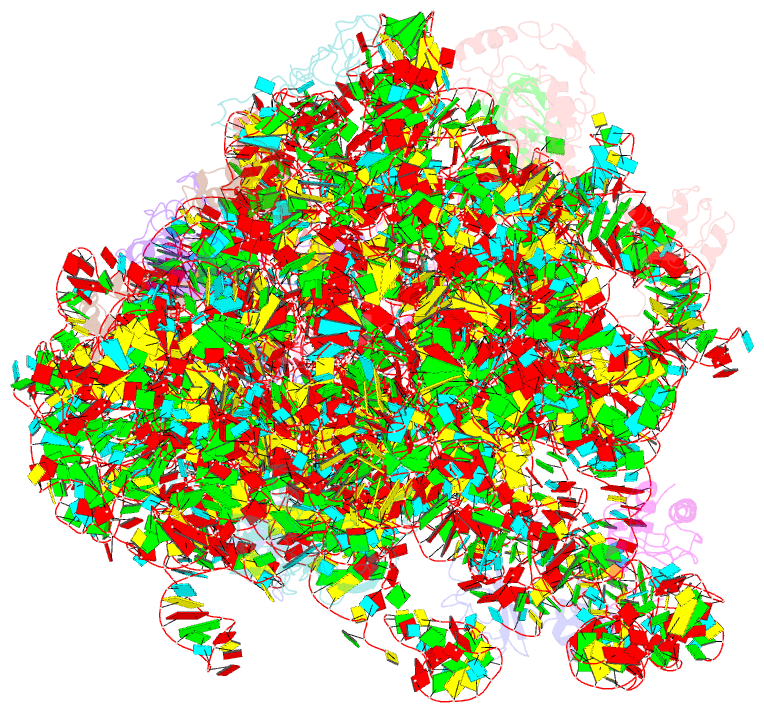
- Summary
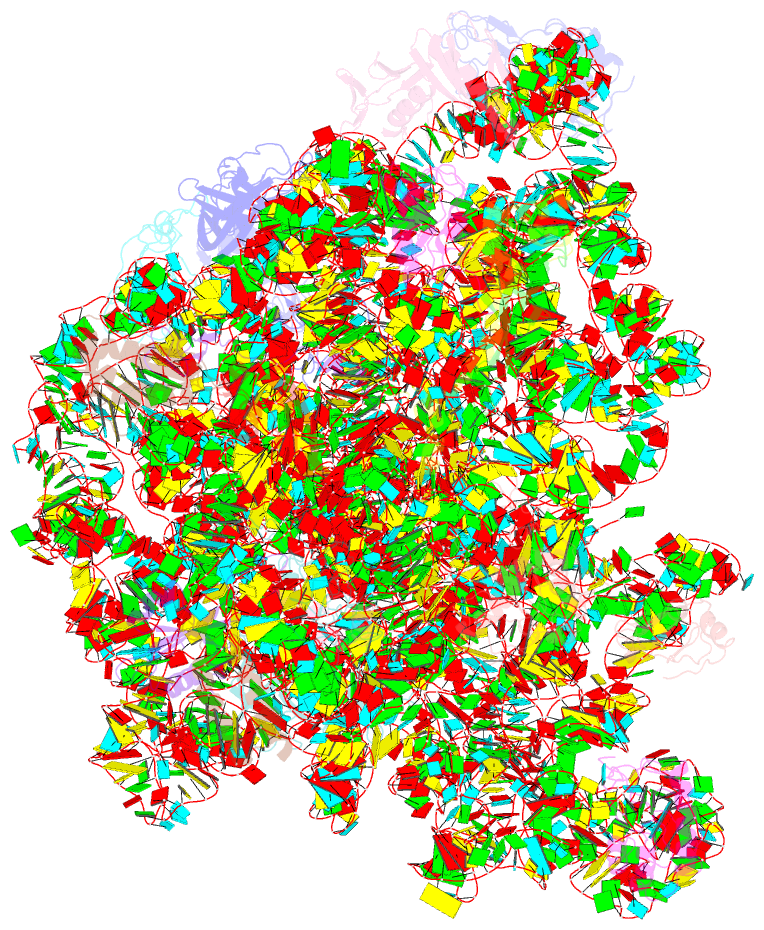
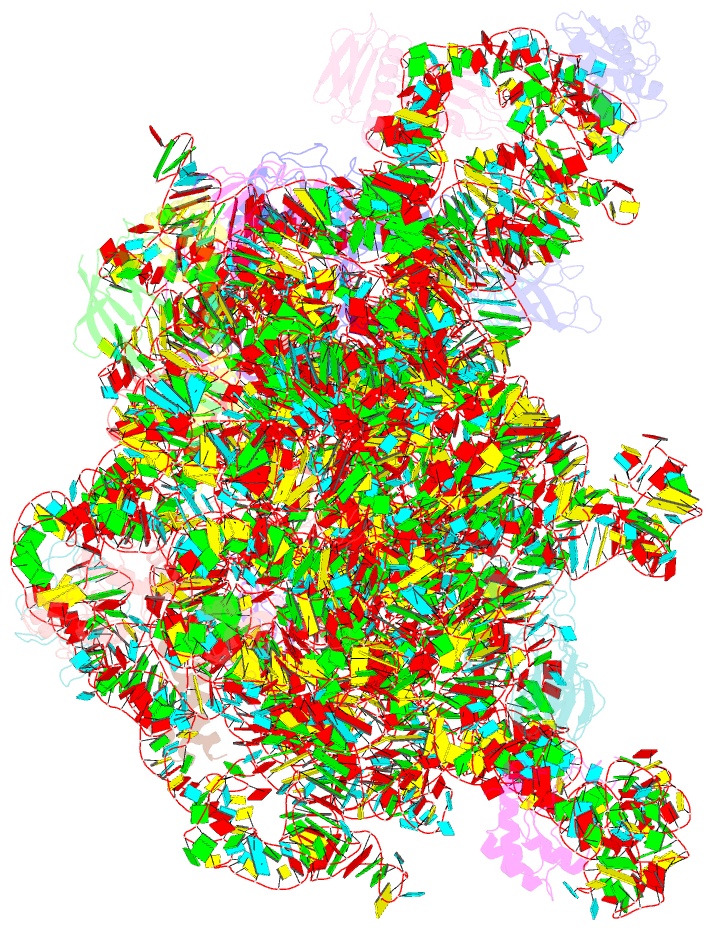
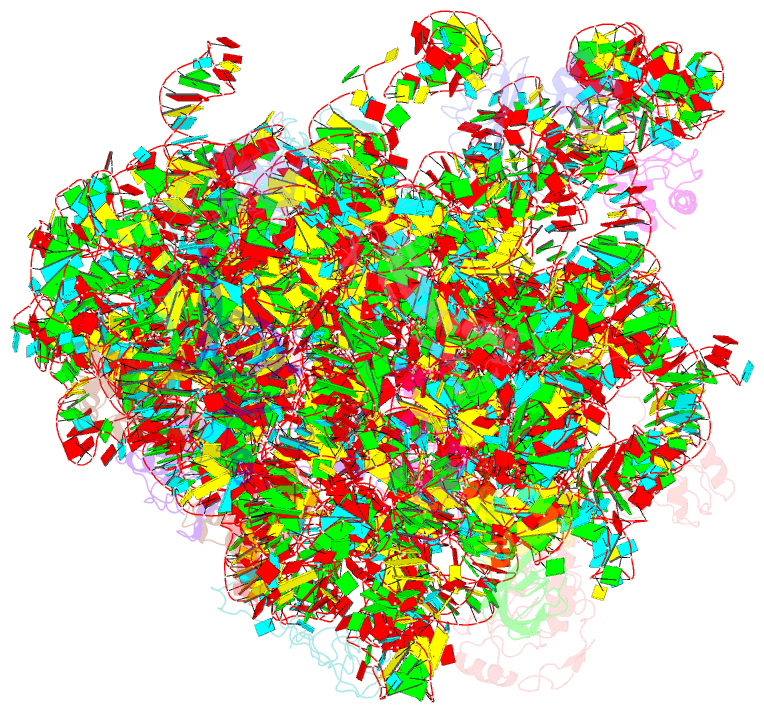
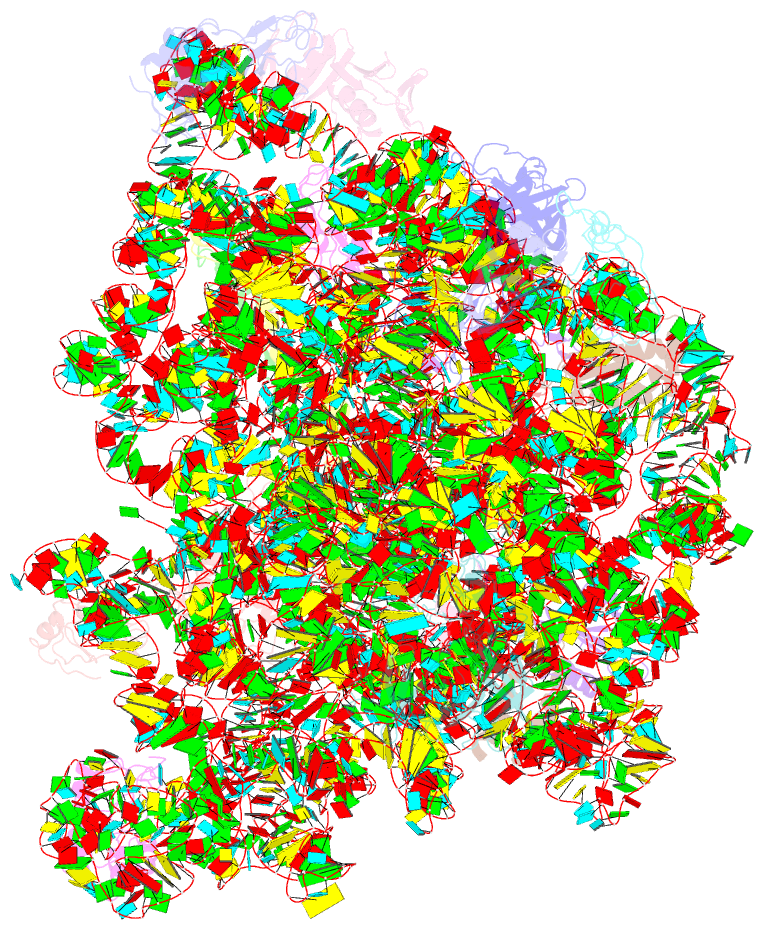
- Atomic model of the immature 50s subunit from bacillus subtilis (state ii-a)
- Reference
- Li N, Chen Y, Guo Q, Zhang Y, Yuan Y, Ma C, Deng H, Lei J, Gao N (2013): "Cryo-EM structures of the late-stage assembly intermediates of the bacterial 50S ribosomal subunit." Nucleic Acids Res., 41, 7073-7083. doi: 10.1093/nar/gkt423.
- Abstract
- Ribosome assembly is a process fundamental for all cellular activities. The efficiency and accuracy of the subunit assembly are tightly regulated and closely monitored. In the present work, we characterized, both compositionally and structurally, a set of in vivo 50S subunit precursors (45S), isolated from a mutant bacterial strain. Our qualitative mass spectrometry data indicate that L28, L16, L33, L36 and L35 are dramatically underrepresented in the 45S particles. This protein spectrum shows interesting similarity to many qualitatively analyzed 50S precursors from different genetic background, indicating the presence of global rate-limiting steps in the late-stage assembly of 50S subunit. Our structural data reveal two major intermediate states for the 45S particles. Consistently, both states severally lack those proteins, but they also differ in the stability of the functional centers of the 50S subunit, demonstrating that they are translationally inactive. Detailed analysis indicates that the orientation of H38 accounts for the global conformational differences in these intermediate structures, and suggests that the reorientation of H38 to its native position is rate-limiting during the late-stage assembly. Especially, H38 plays an essential role in stabilizing the central protuberance, through the interaction with the 5S rRNA, and the correctly orientated H38 is likely a prerequisite for further maturation of the 50S subunit.