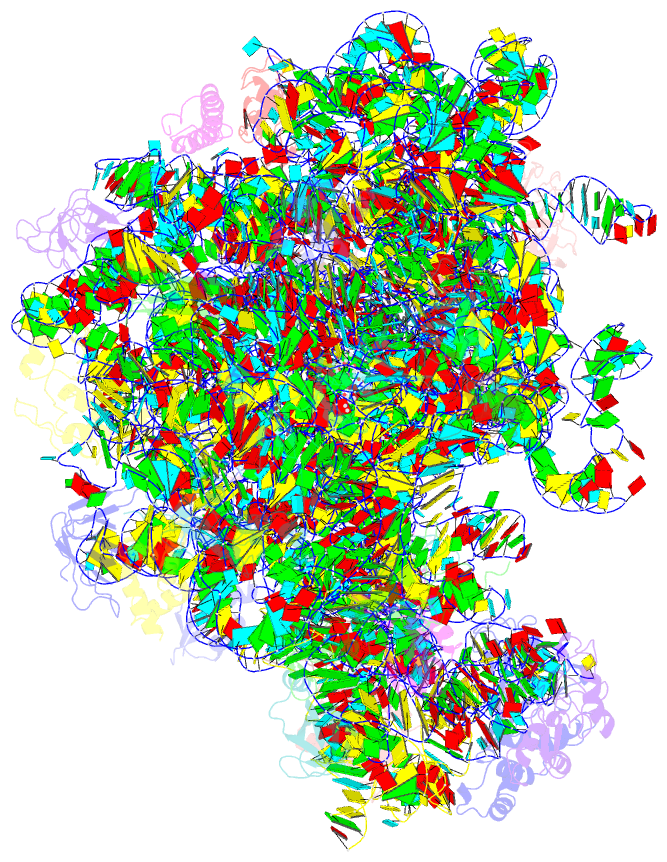
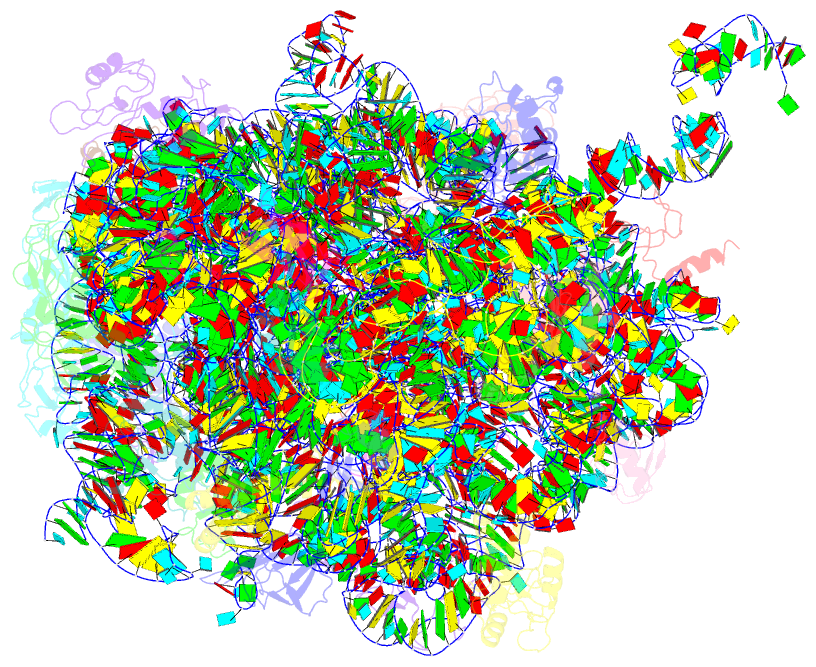
Summary information and primary citation
- PDB-id
- 3j5l; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome-antibiotic
- Method
- cryo-EM (6.6 Å)
- Summary
- Structure of the e. coli 50s subunit with ermbl nascent chain
- Reference
- Arenz S, Ramu H, Gupta P, Berninghausen O, Beckmann R, Vazquez-Laslop N, Mankin AS, Wilson DN (2014): "Molecular basis for erythromycin-dependent ribosome stalling during translation of the ErmBL leader peptide." Nat Commun, 5, 3501. doi: 10.1038/ncomms4501.
- Abstract
- In bacteria, ribosome stalling during translation of ErmBL leader peptide occurs in the presence of the antibiotic erythromycin and leads to induction of expression of the downstream macrolide resistance methyltransferase ErmB. The lack of structures of drug-dependent stalled ribosome complexes (SRCs) has limited our mechanistic understanding of this regulatory process. Here we present a cryo-electron microscopy structure of the erythromycin-dependent ErmBL-SRC. The structure reveals that the antibiotic does not interact directly with ErmBL, but rather redirects the path of the peptide within the tunnel. Furthermore, we identify a key peptide-ribosome interaction that defines an important relay pathway from the ribosomal tunnel to the peptidyltransferase centre (PTC). The PTC of the ErmBL-SRC appears to adopt an uninduced state that prevents accommodation of Lys-tRNA at the A-site, thus providing structural basis for understanding how the drug and the nascent peptide cooperate to inhibit peptide bond formation and induce translation arrest.