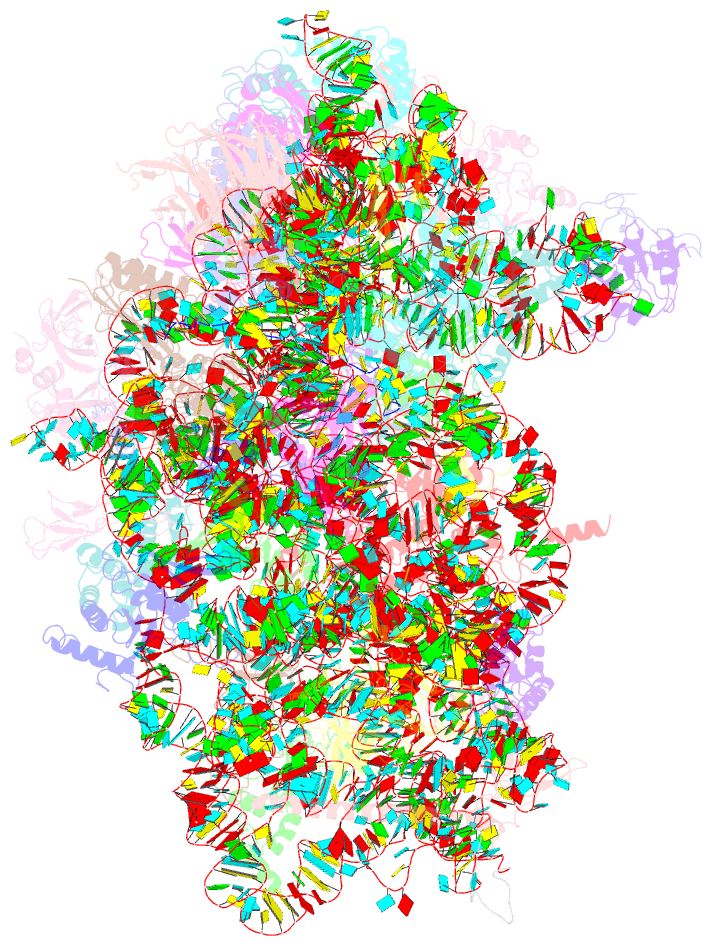
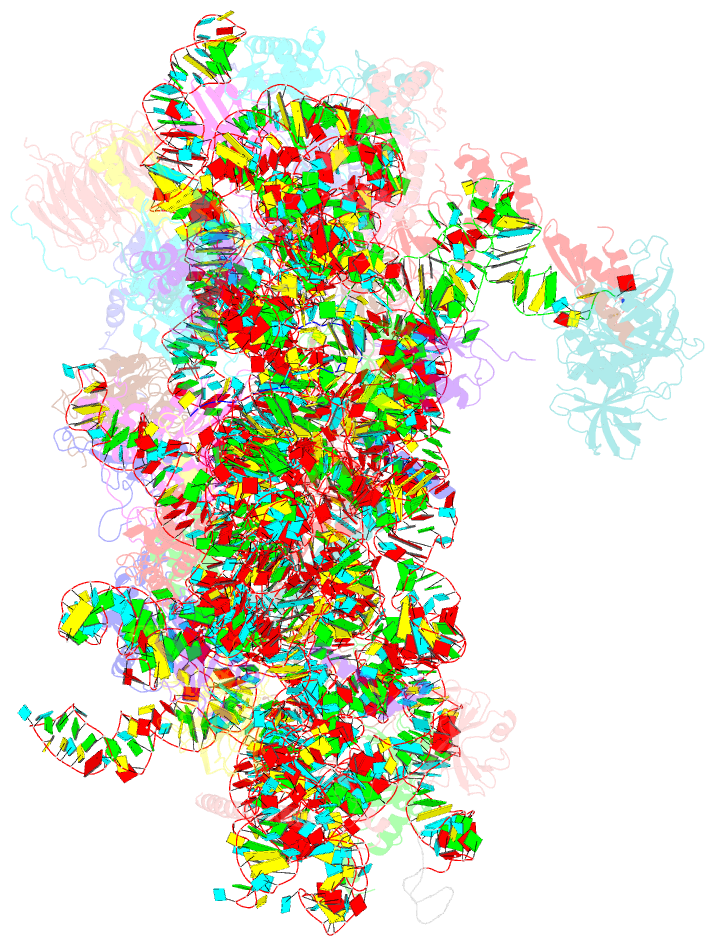
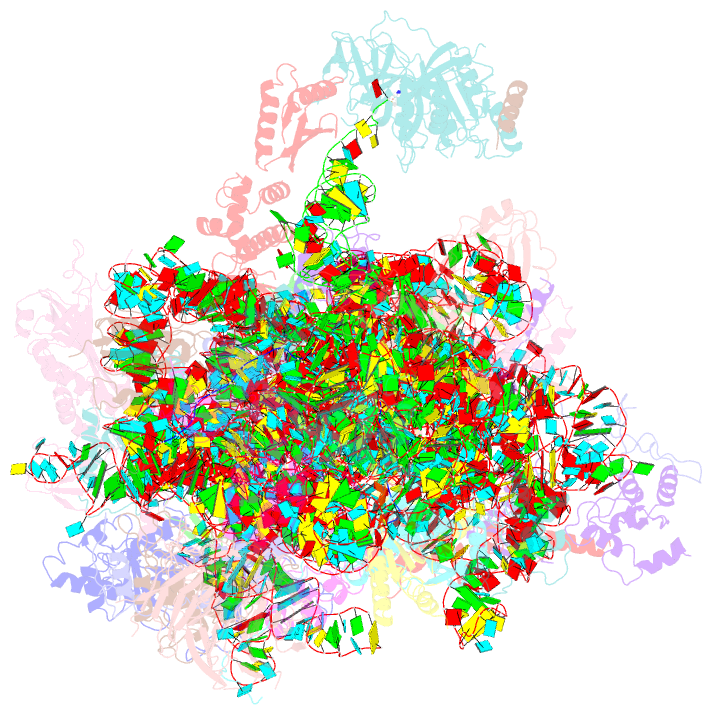
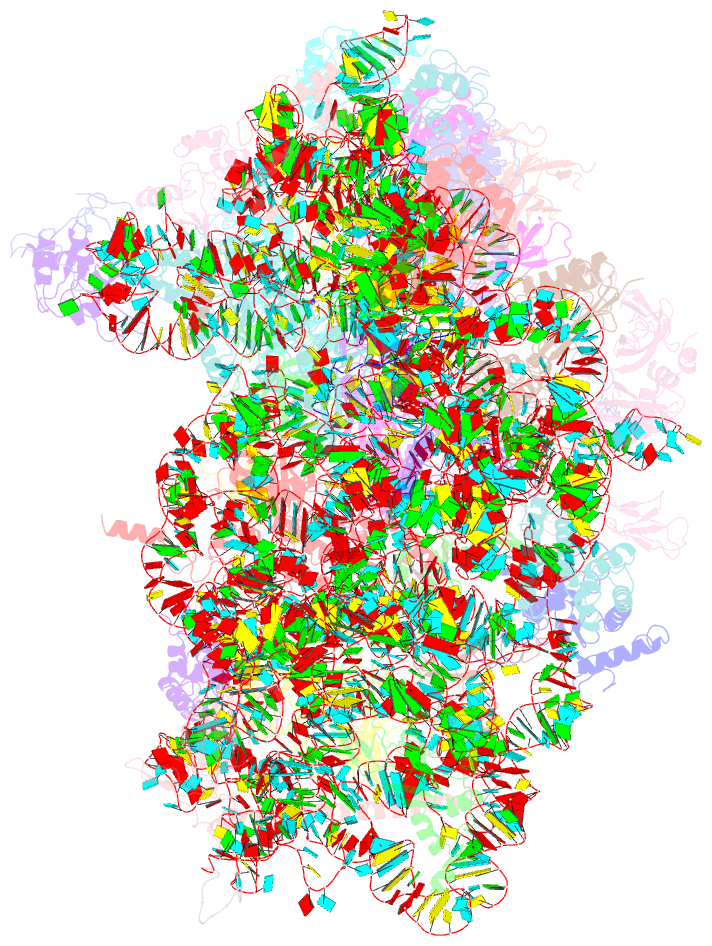
Summary information and primary citation
- PDB-id
- 3j81; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (4.0 Å)
- Summary
- Cryoem structure of a partial yeast 48s preinitiation complex
- Reference
- Hussain T, Llacer JL, Fernandez IS, Munoz A, Martin-Marcos P, Savva CG, Lorsch JR, Hinnebusch AG, Ramakrishnan V (2014): "Structural changes enable start codon recognition by the eukaryotic translation initiation complex." Cell(Cambridge,Mass.), 159, 597-607. doi: 10.1016/j.cell.2014.10.001.
- Abstract
- During eukaryotic translation initiation, initiator tRNA does not insert fully into the P decoding site on the 40S ribosomal subunit. This conformation (POUT) is compatible with scanning mRNA for the AUG start codon. Base pairing with AUG is thought to promote isomerization to a more stable conformation (PIN) that arrests scanning and promotes dissociation of eIF1 from the 40S subunit. Here, we present a cryoEM reconstruction of a yeast preinitiation complex at 4.0 Å resolution with initiator tRNA in the PIN state, prior to eIF1 release. The structure reveals stabilization of the codon-anticodon duplex by the N-terminal tail of eIF1A, changes in the structure of eIF1 likely instrumental in its subsequent release, and changes in the conformation of eIF2. The mRNA traverses the entire mRNA cleft and makes connections to the regulatory domain of eIF2?, eIF1A, and ribosomal elements that allow recognition of context nucleotides surrounding the AUG codon.