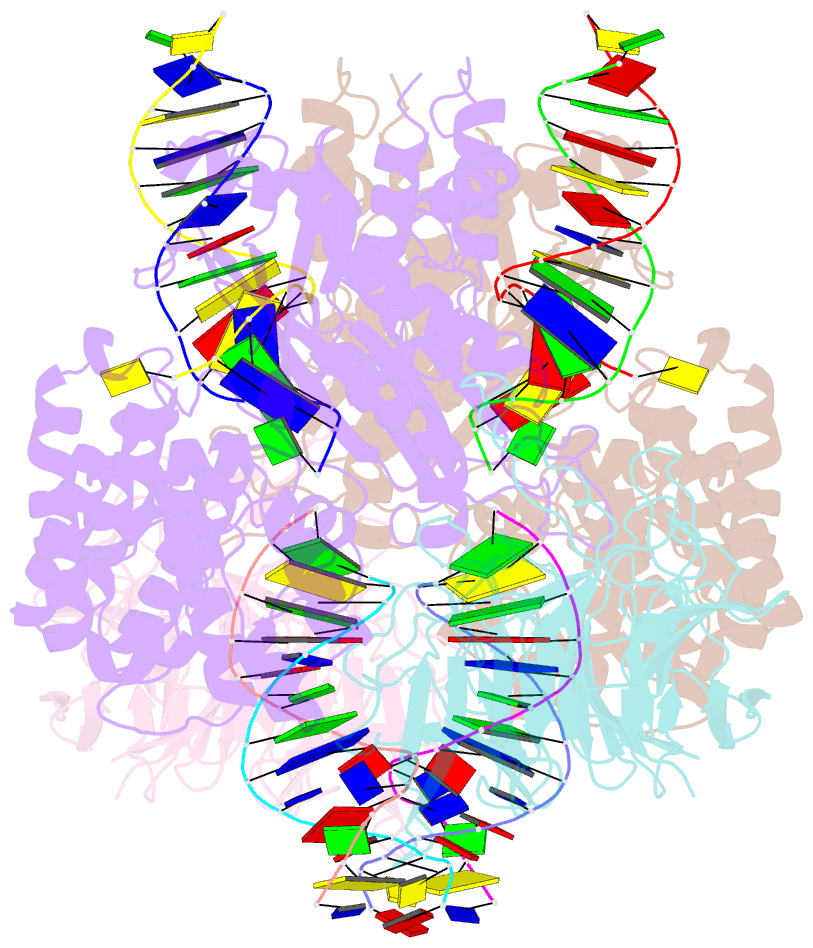
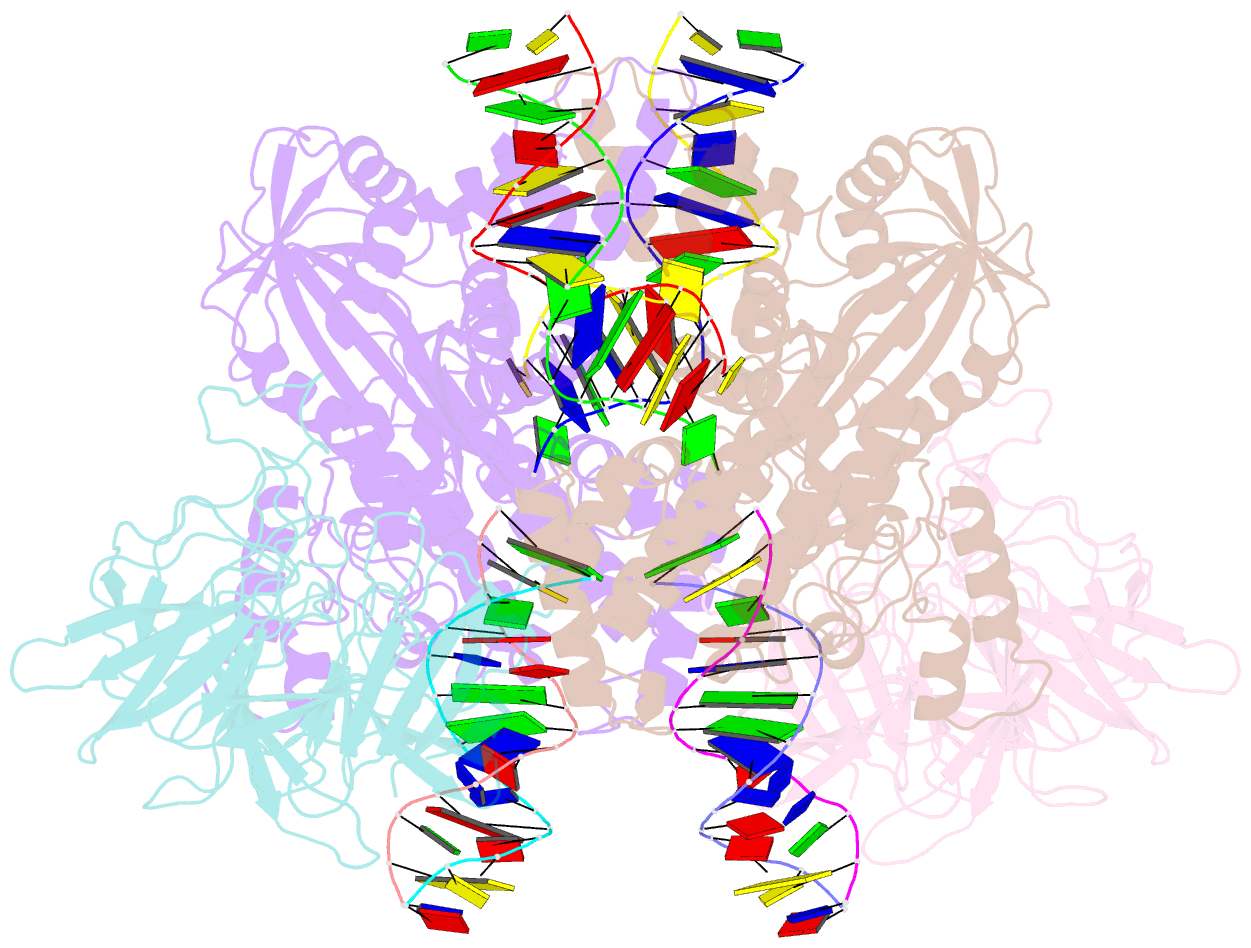
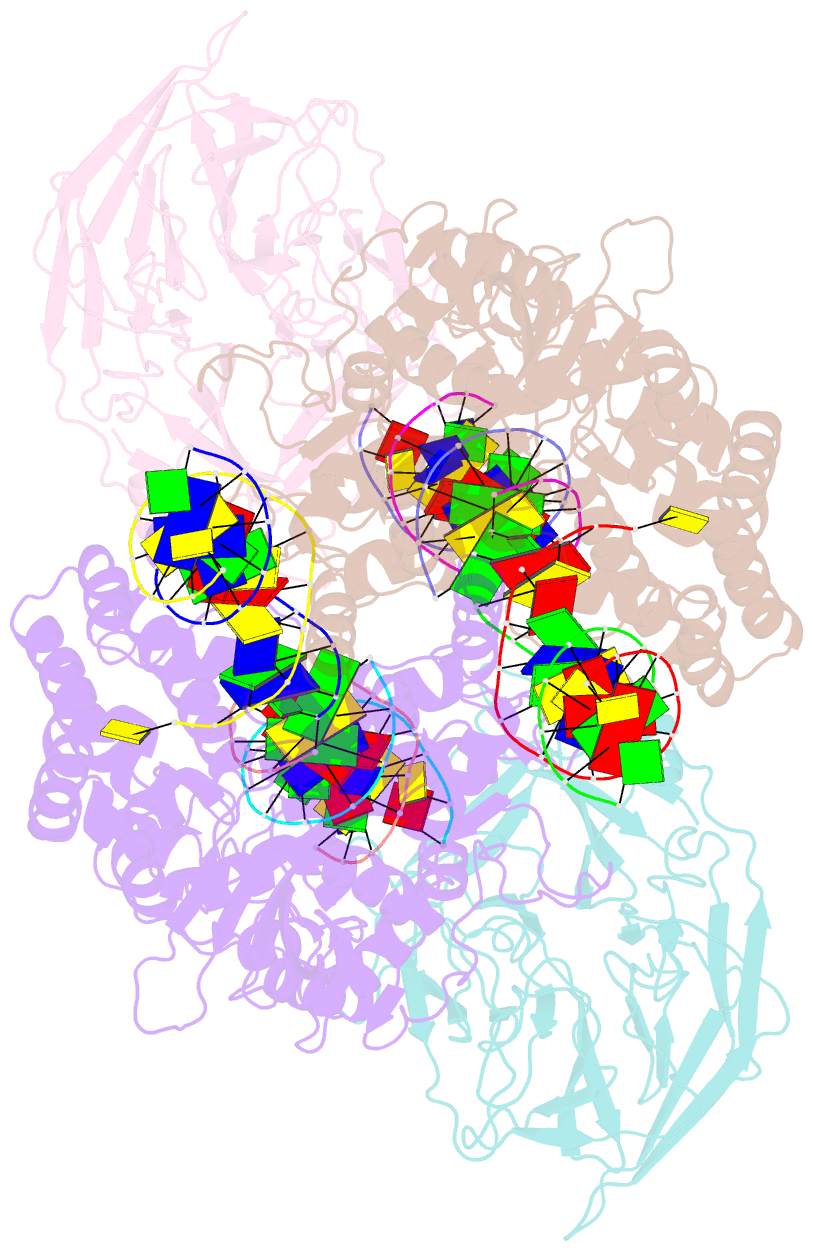
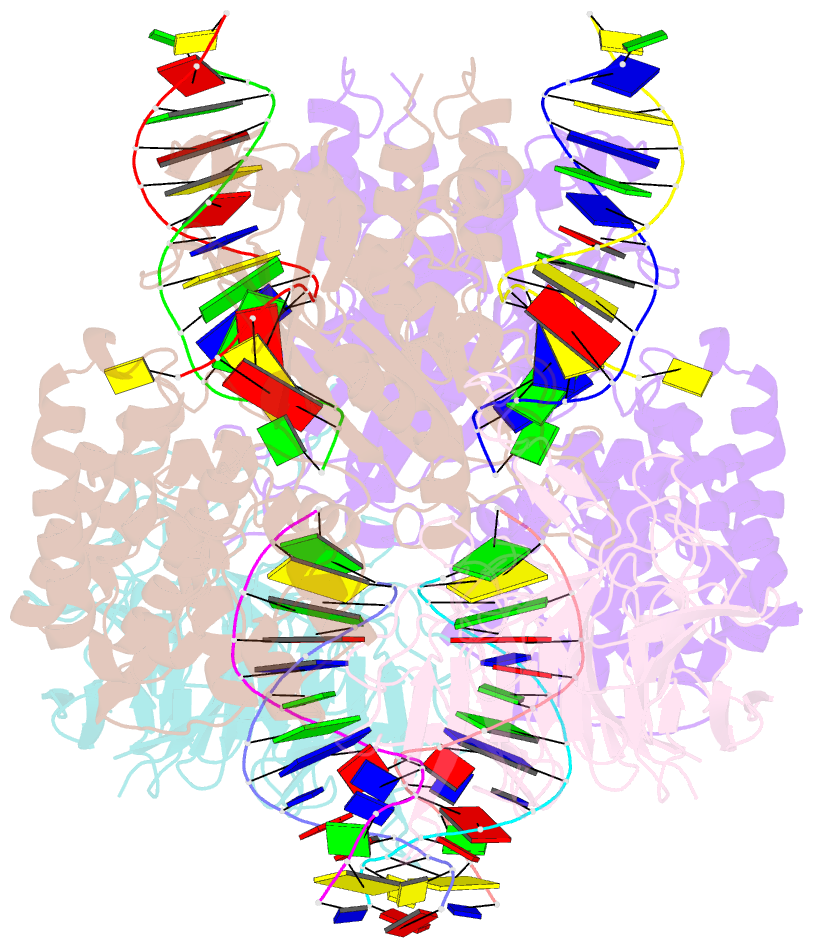
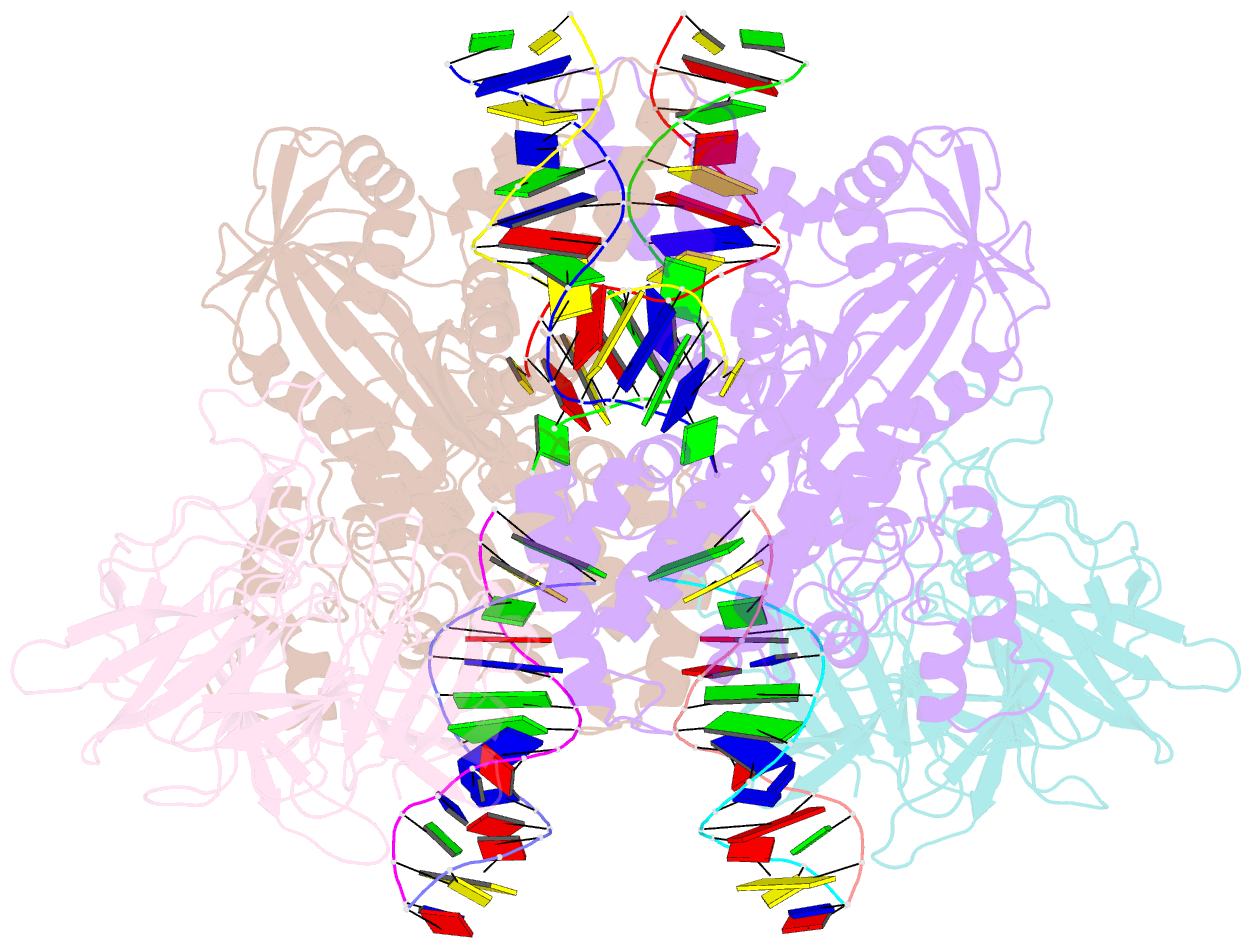
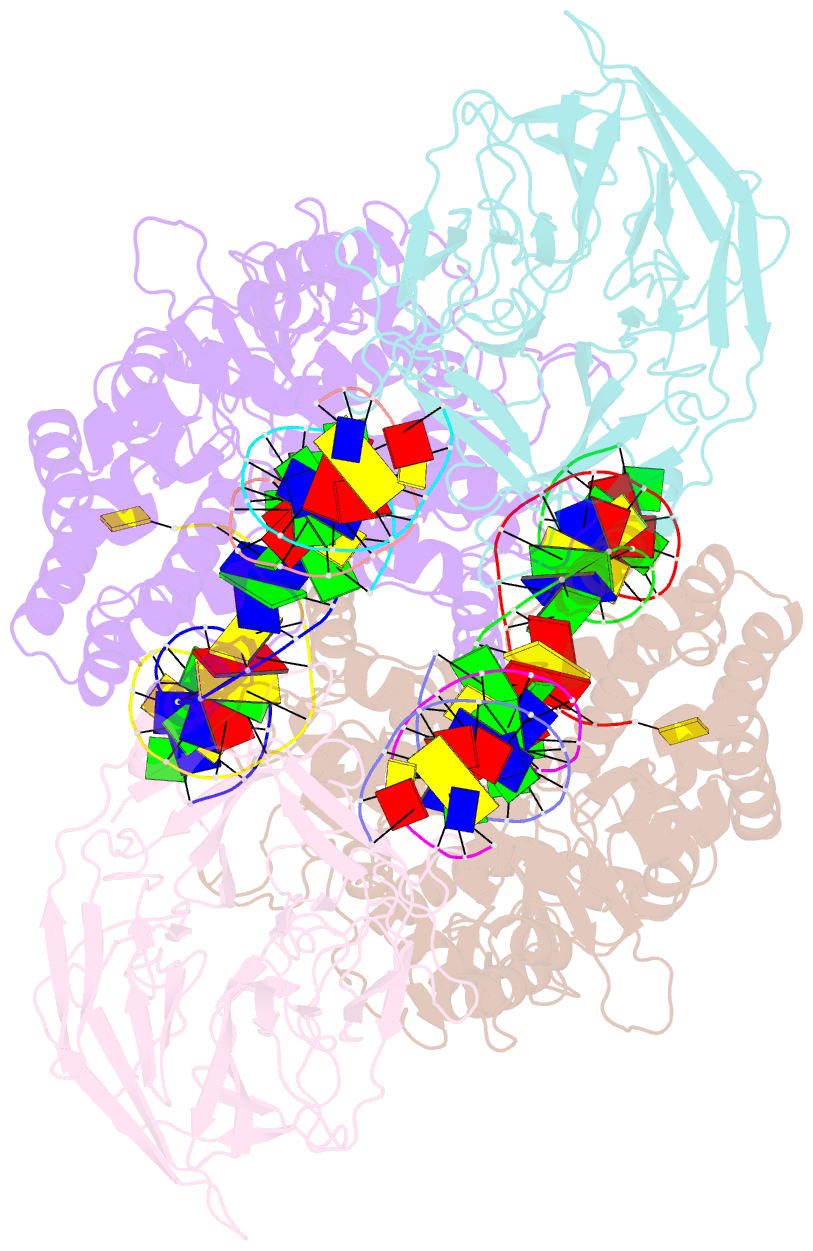
Summary information and primary citation
- PDB-id
- 3jbx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- recombination-DNA
- Method
- cryo-EM (3.4 Å)
- Summary
- Cryo-electron microscopy structure of rag signal end complex (c2 symmetry)
- Reference
- Ru H, Chambers MG, Fu TM, Tong AB, Liao M, Wu H (2015): "Molecular Mechanism of V(D)J Recombination from Synaptic RAG1-RAG2 Complex Structures." Cell(Cambridge,Mass.), 163, 1138-1152. doi: 10.1016/j.cell.2015.10.055.
- Abstract
- Diverse repertoires of antigen-receptor genes that result from combinatorial splicing of coding segments by V(D)J recombination are hallmarks of vertebrate immunity. The (RAG1-RAG2)2 recombinase (RAG) recognizes recombination signal sequences (RSSs) containing a heptamer, a spacer of 12 or 23 base pairs, and a nonamer (12-RSS or 23-RSS) and introduces precise breaks at RSS-coding segment junctions. RAG forms synaptic complexes only with one 12-RSS and one 23-RSS, a dogma known as the 12/23 rule that governs the recombination fidelity. We report cryo-electron microscopy structures of synaptic RAG complexes at up to 3.4 Å resolution, which reveal a closed conformation with base flipping and base-specific recognition of RSSs. Distortion at RSS-coding segment junctions and base flipping in coding segments uncover the two-metal-ion catalytic mechanism. Induced asymmetry involving tilting of the nonamer-binding domain dimer of RAG1 upon binding of HMGB1-bent 12-RSS or 23-RSS underlies the molecular mechanism for the 12/23 rule.