Summary information and primary citation
- PDB-id
- 3jsp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.9 Å)
- Summary
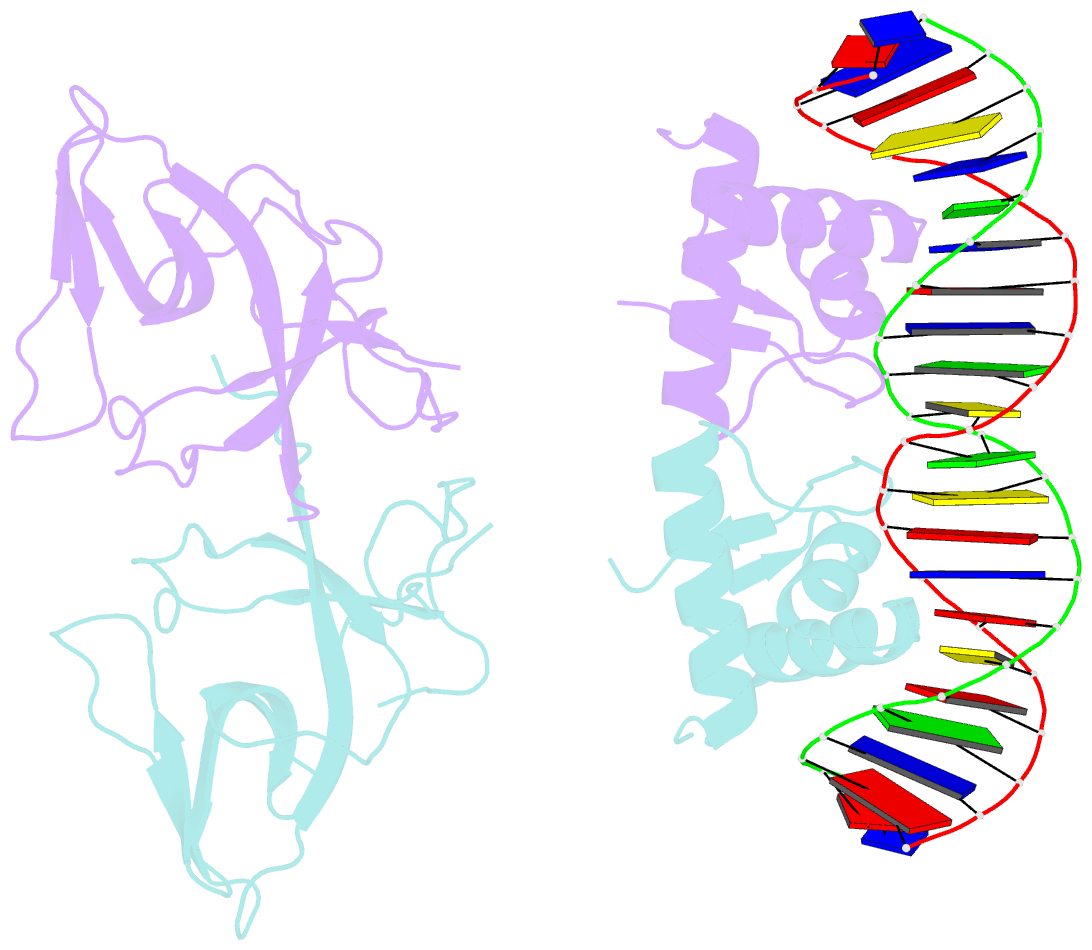
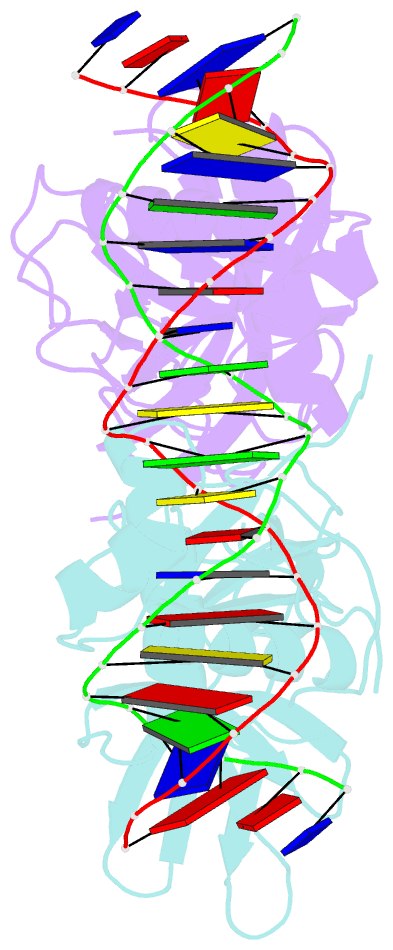
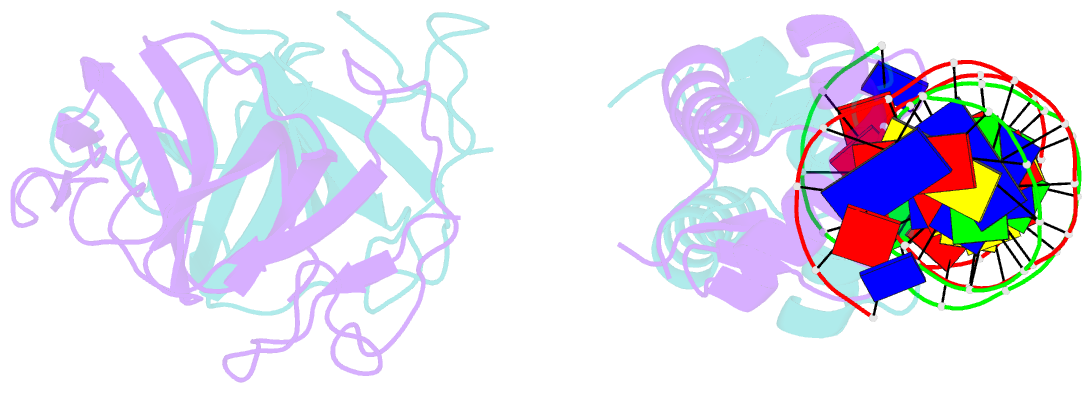
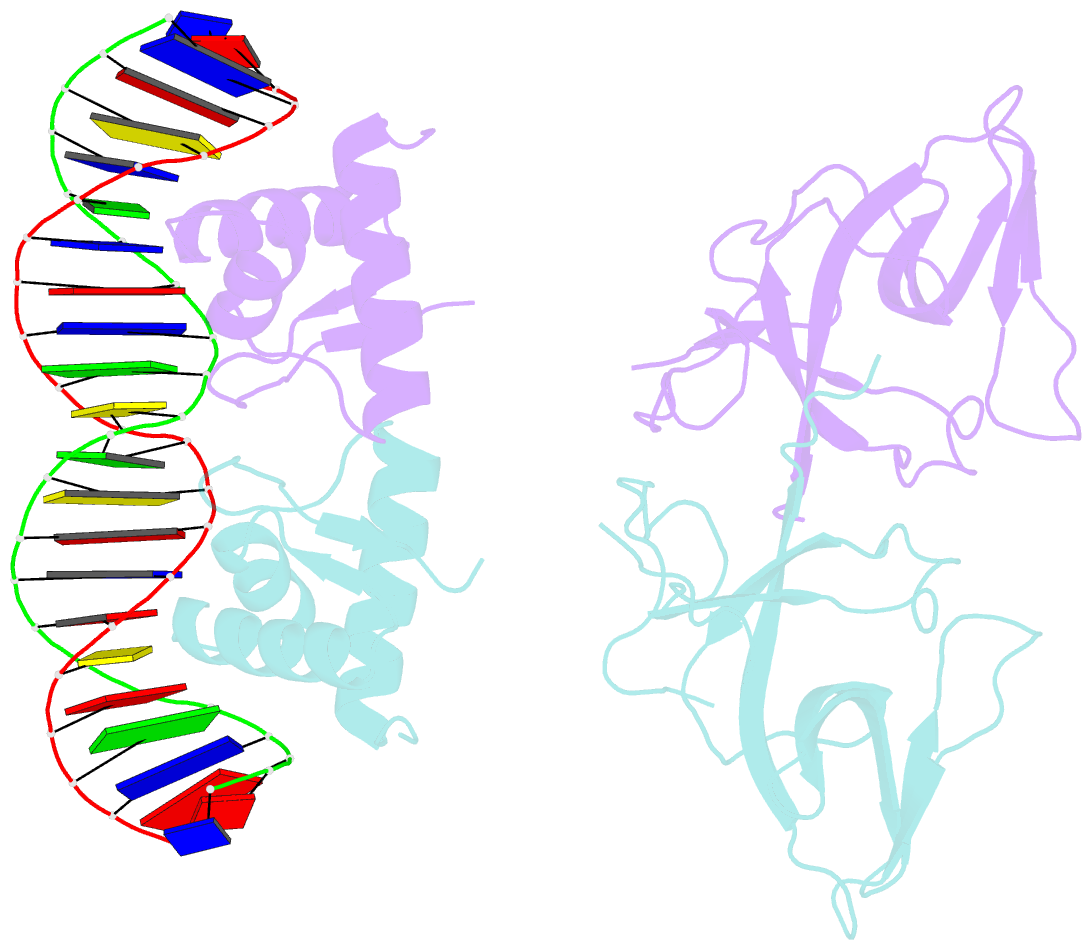
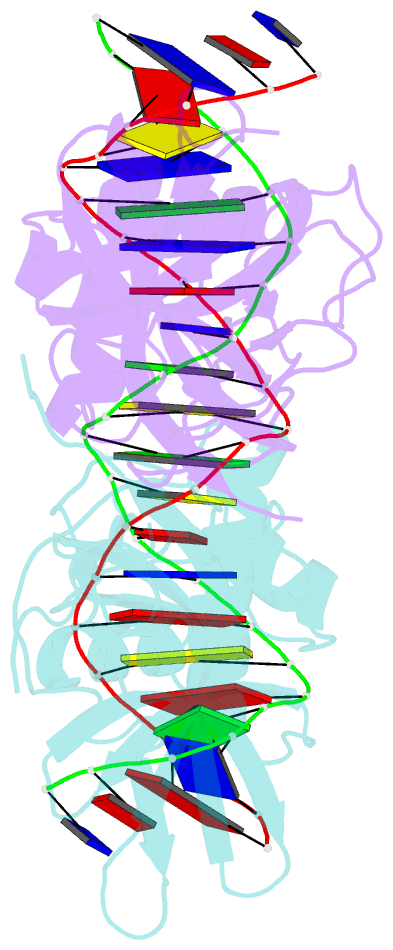
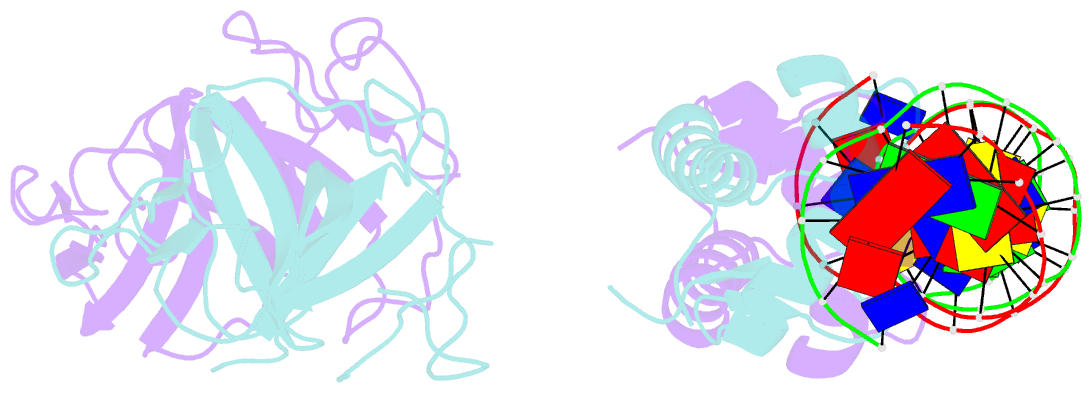
- Classic protein with a new twist: crystal structure of a lexa repressor DNA complex
- Reference
- Zhang AP, Pigli YZ, Rice PA (2010): "Structure of the LexA-DNA complex and implications for SOS box measurement." Nature, 466, 883-886. doi: 10.1038/nature09200.
- Abstract
- The eubacterial SOS system is a paradigm of cellular DNA damage and repair, and its activation can contribute to antibiotic resistance. Under normal conditions, LexA represses the transcription of many DNA repair proteins by binding to SOS 'boxes' in their operators. Under genotoxic stress, accumulating complexes of RecA, ATP and single-stranded DNA (ssDNA) activate LexA for autocleavage. To address how LexA recognizes its binding sites, we determined three crystal structures of Escherichia coli LexA in complex with SOS boxes. Here we report the structure of these LexA-DNA complexes. The DNA-binding domains of the LexA dimer interact with the DNA in the classical fashion of a winged helix-turn-helix motif. However, the wings of these two DNA-binding domains bind to the same minor groove of the DNA. These wing-wing contacts may explain why the spacing between the two half-sites of E. coli SOS boxes is invariant.