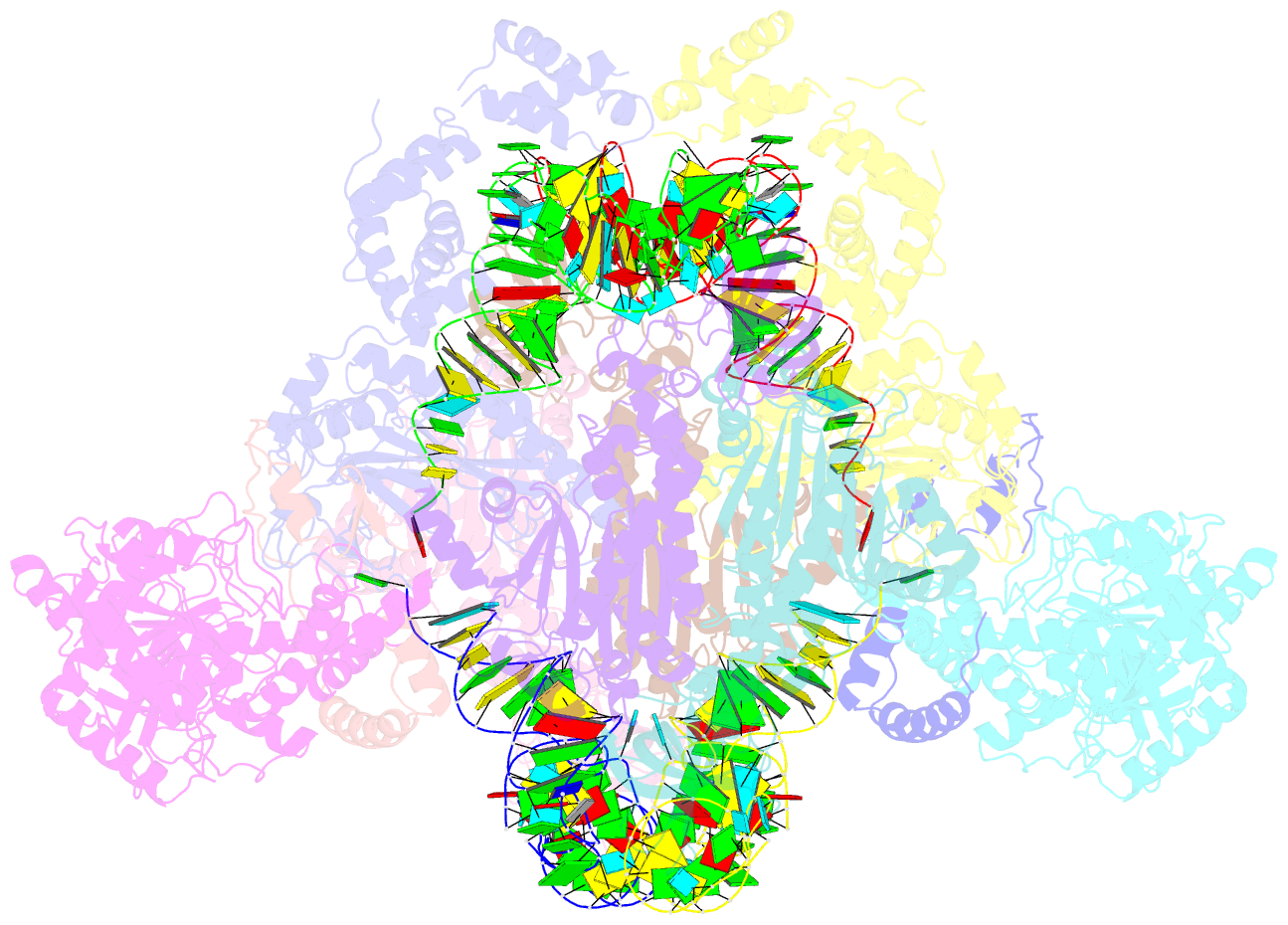
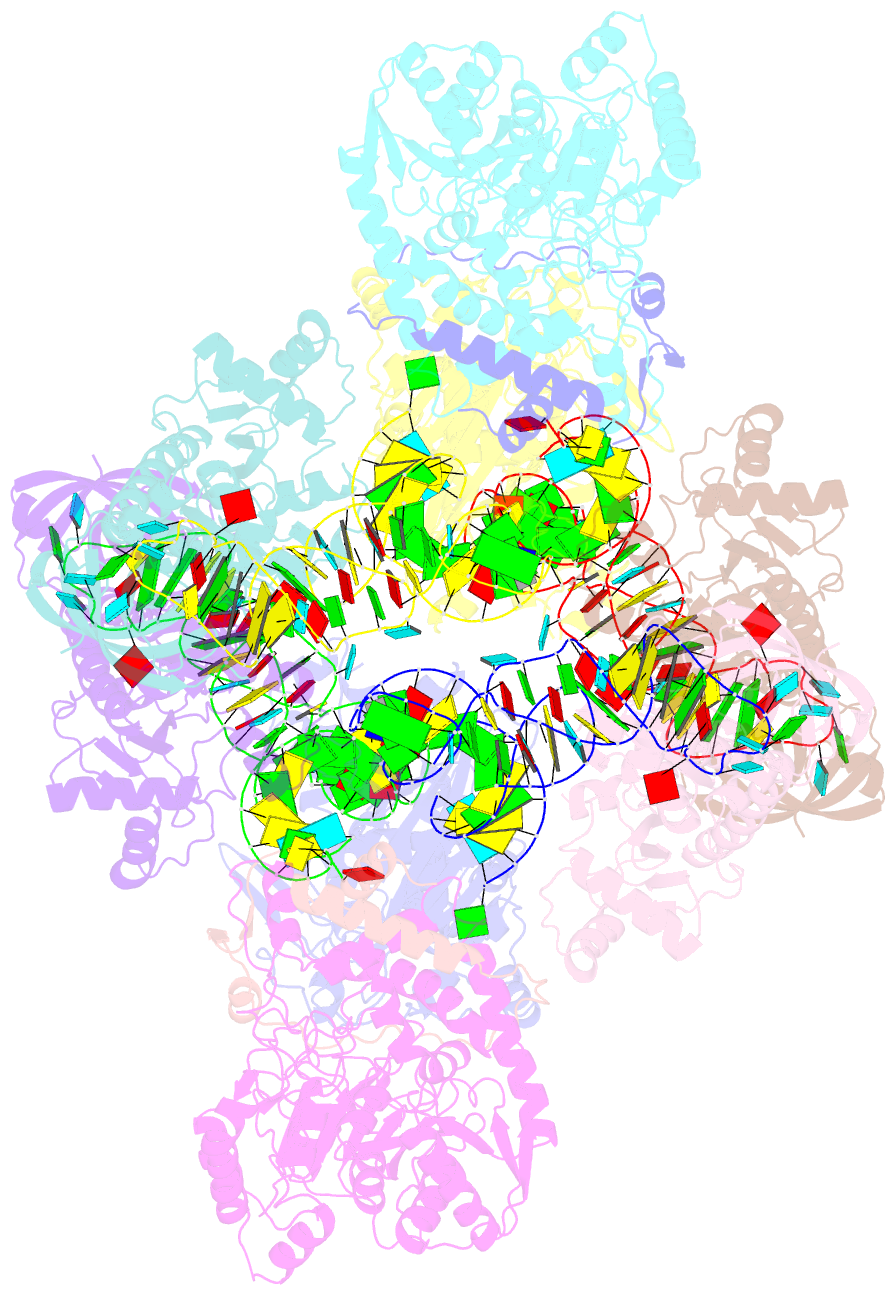
Summary information and primary citation
- PDB-id
- 3kfu; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-RNA
- Method
- X-ray (3.0 Å)
- Summary
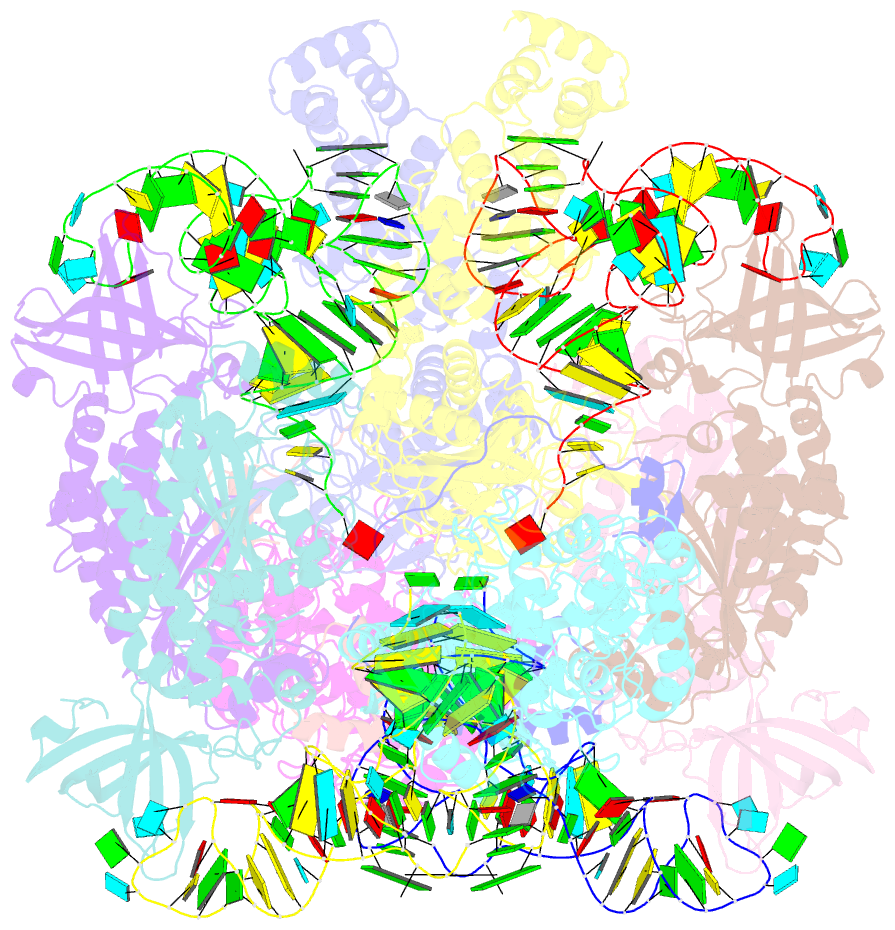
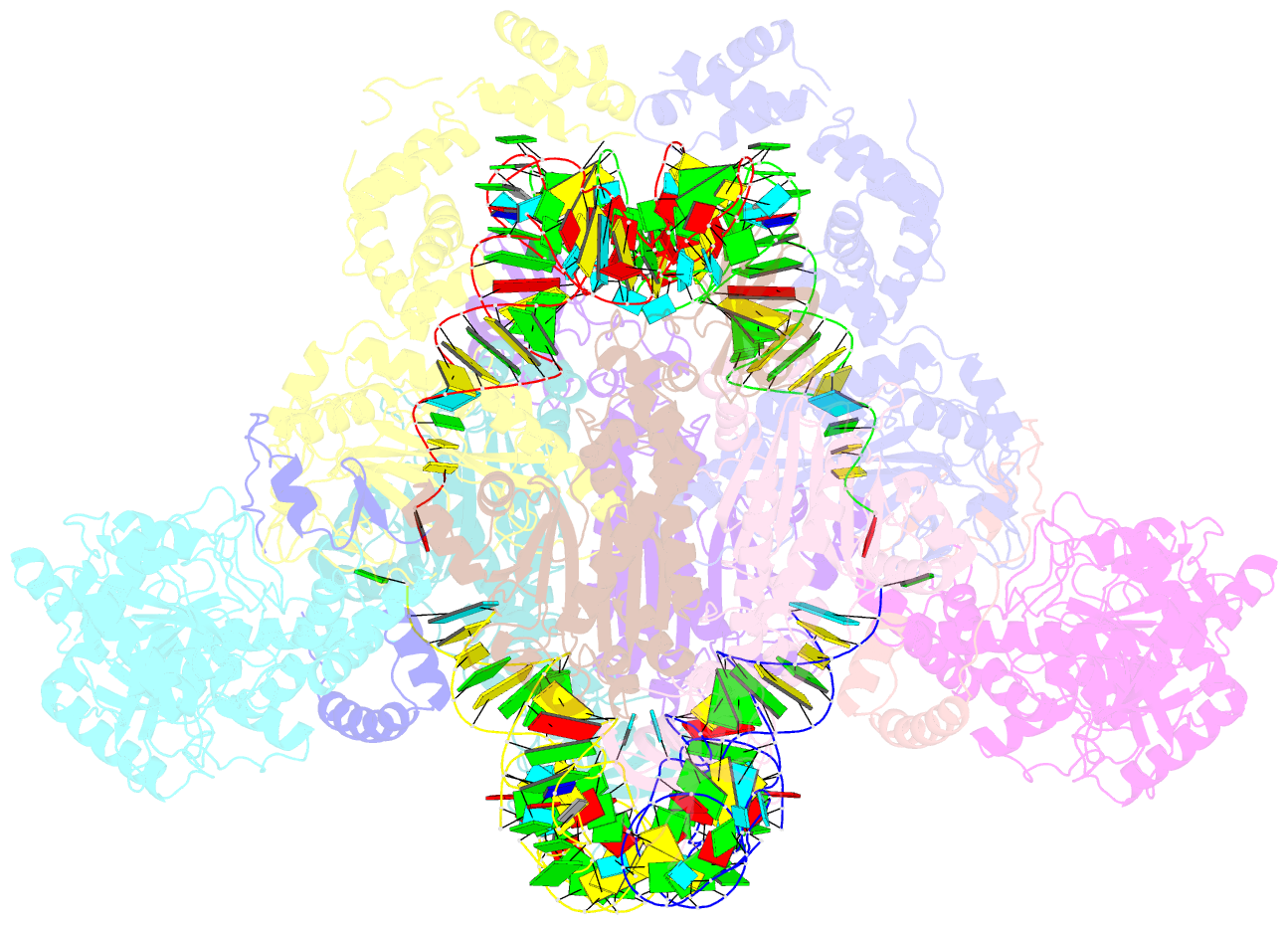
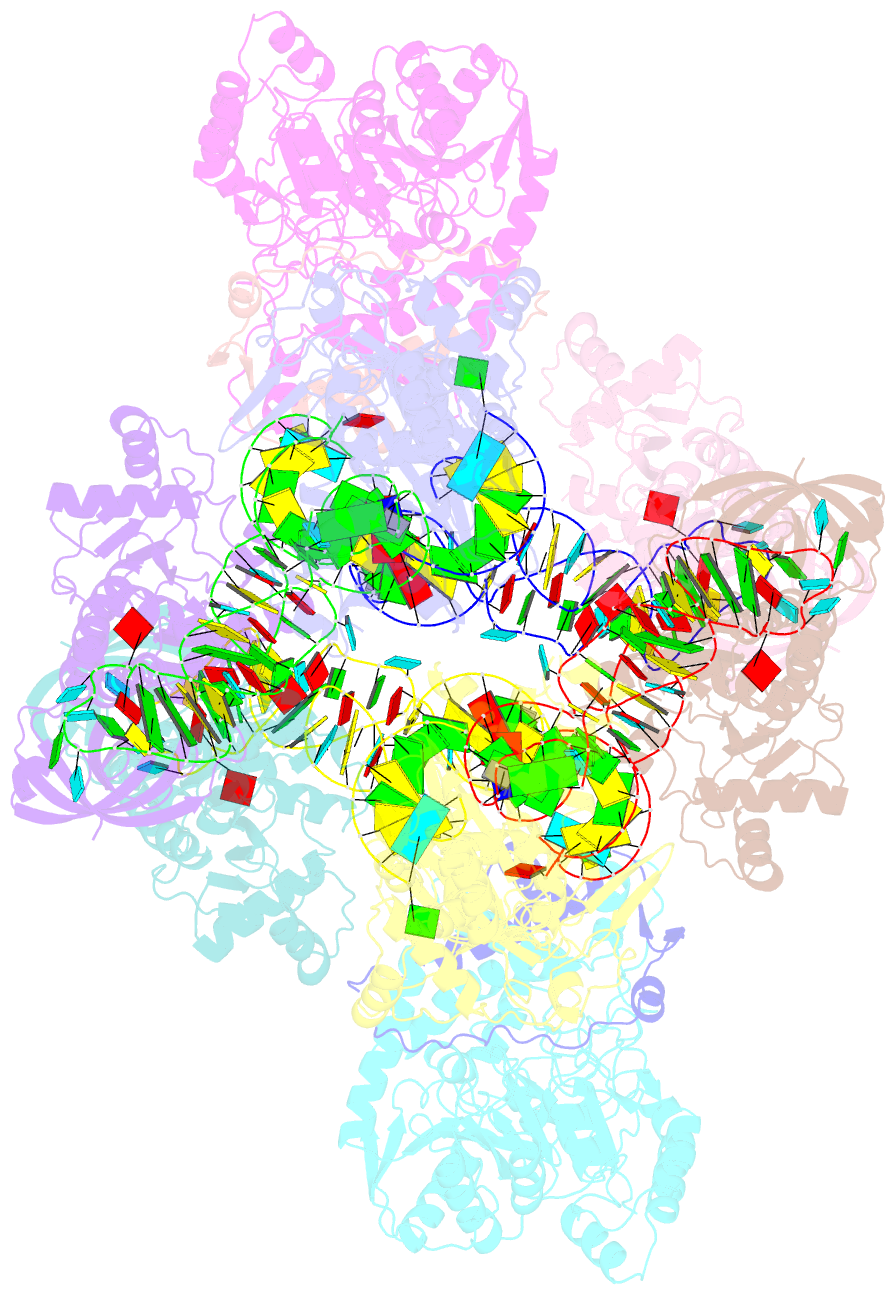
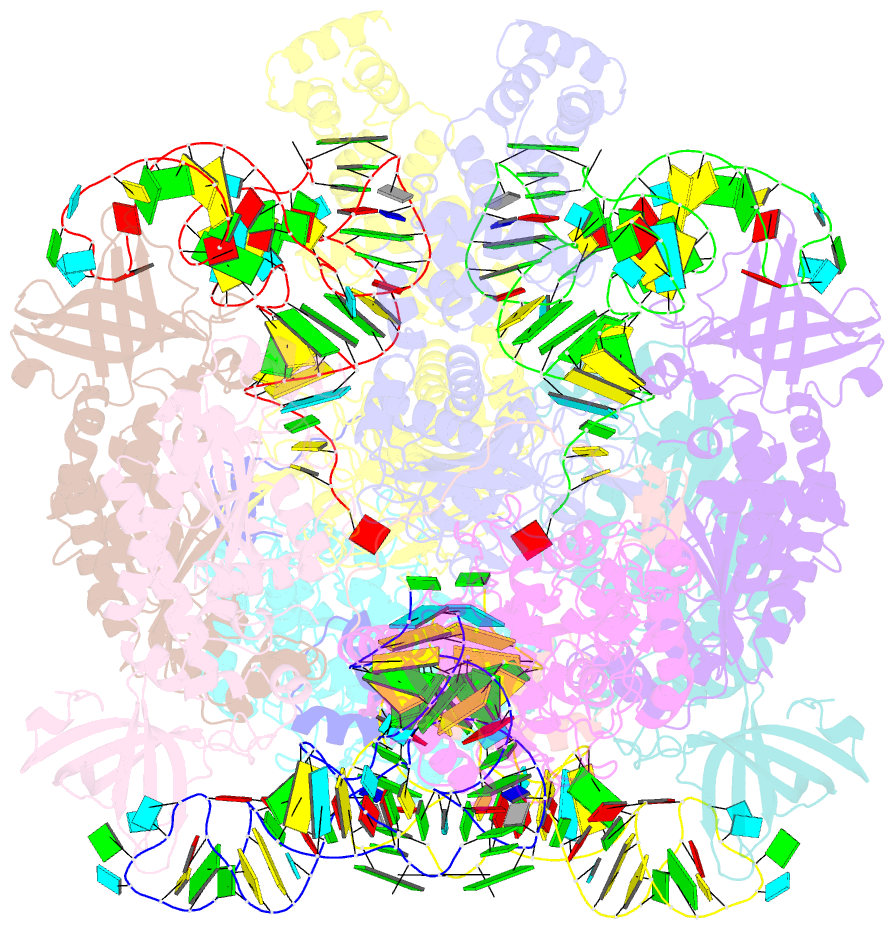
- Crystal structure of the transamidosome
- Reference
- Blaise M, Bailly M, Frechin M, Behrens MA, Fischer F, Oliveira CL, Becker HD, Pedersen JS, Thirup S, Kern D (2010): "Crystal structure of a transfer-ribonucleoprotein particle that promotes asparagine formation." Embo J., 29, 3118-3129. doi: 10.1038/emboj.2010.192.
- Abstract
- Four out of the 22 aminoacyl-tRNAs (aa-tRNAs) are systematically or alternatively synthesized by an indirect, two-step route requiring an initial mischarging of the tRNA followed by tRNA-dependent conversion of the non-cognate amino acid. During tRNA-dependent asparagine formation, tRNA(Asn) promotes assembly of a ribonucleoprotein particle called transamidosome that allows channelling of the aa-tRNA from non-discriminating aspartyl-tRNA synthetase active site to the GatCAB amidotransferase site. The crystal structure of the Thermus thermophilus transamidosome determined at 3 A resolution reveals a particle formed by two GatCABs, two dimeric ND-AspRSs and four tRNAs(Asn) molecules. In the complex, only two tRNAs are bound in a functional state, whereas the two other ones act as an RNA scaffold enabling release of the asparaginyl-tRNA(Asn) without dissociation of the complex. We propose that the crystal structure represents a transient state of the transamidation reaction. The transamidosome constitutes a transfer-ribonucleoprotein particle in which tRNAs serve the function of both substrate and structural foundation for a large molecular machine.