Summary information and primary citation
- PDB-id
- 3kjo; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (1.8 Å)
- Summary
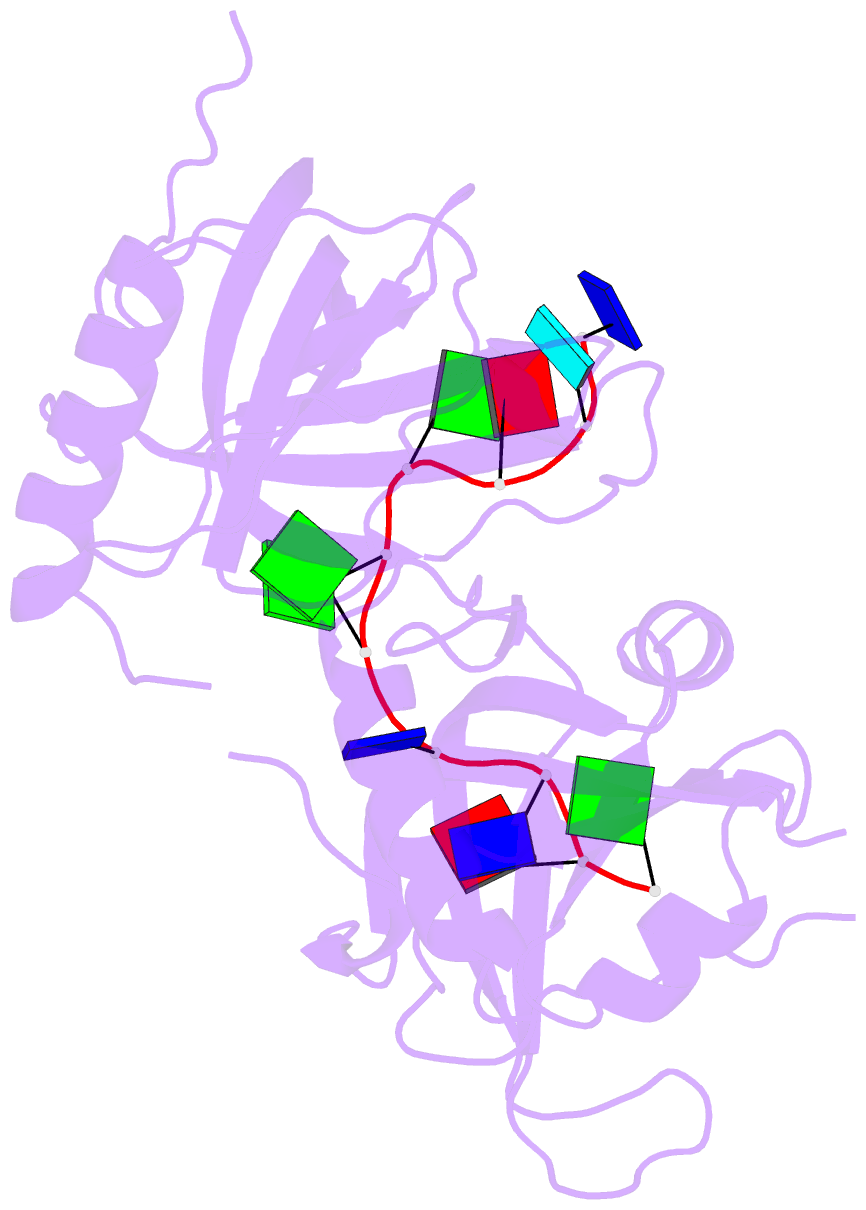
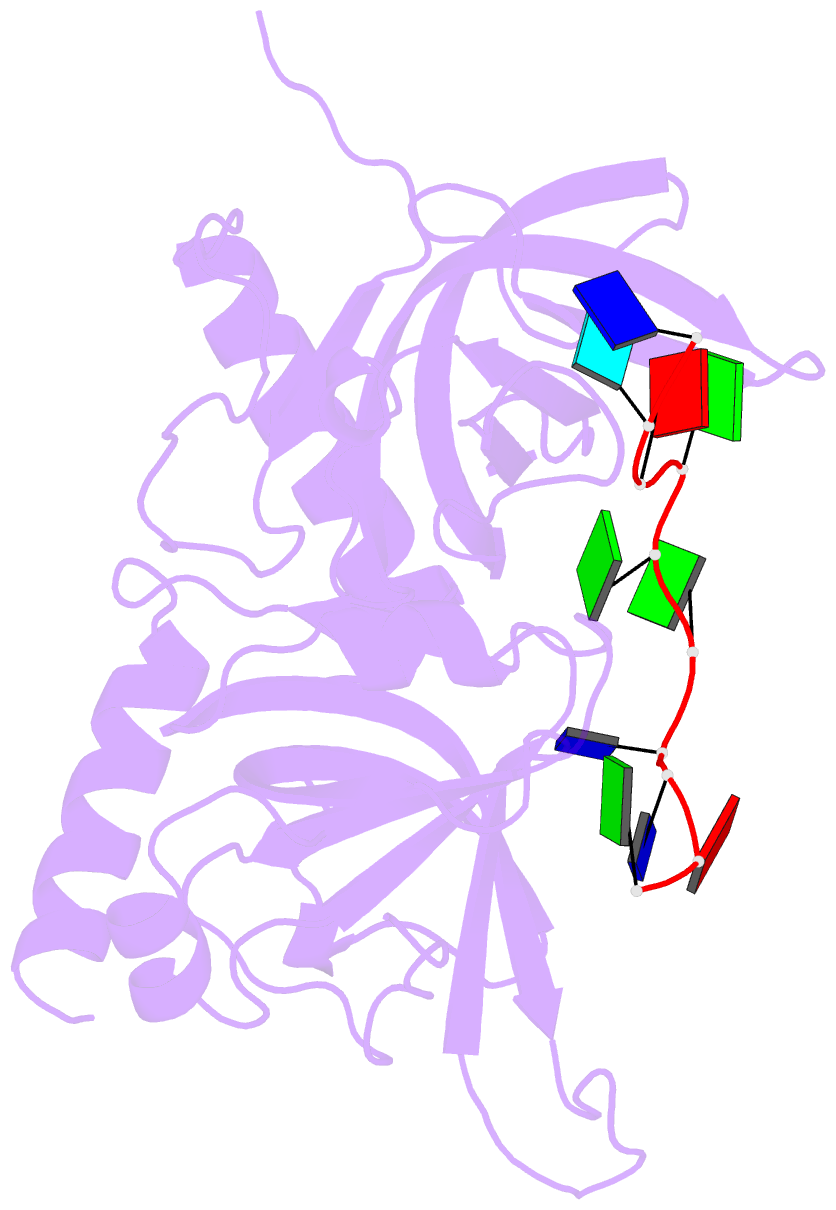
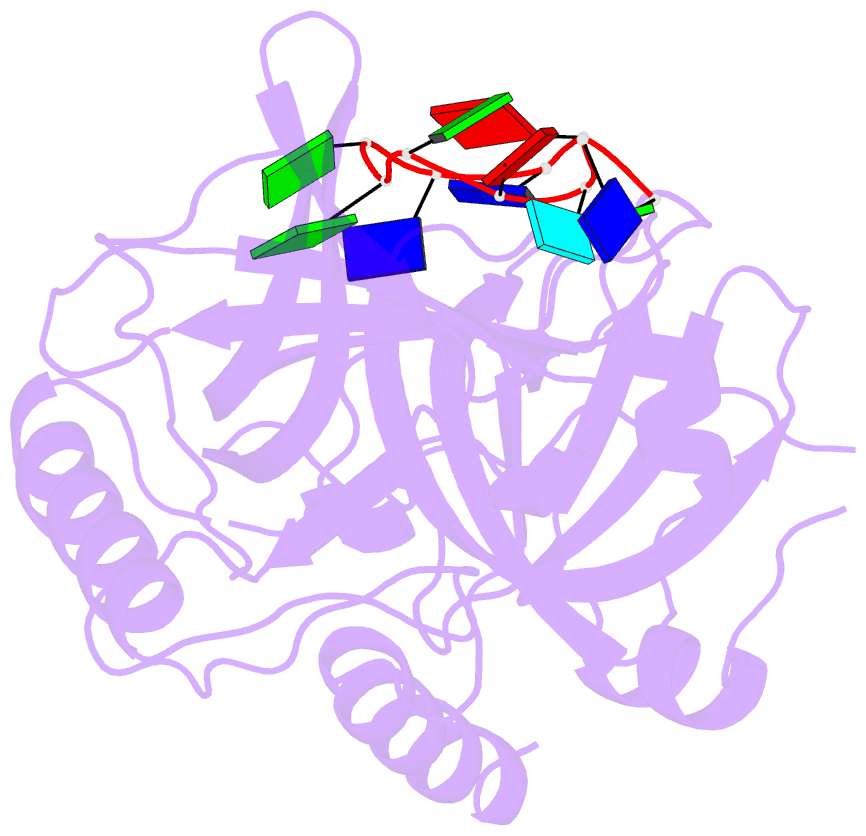
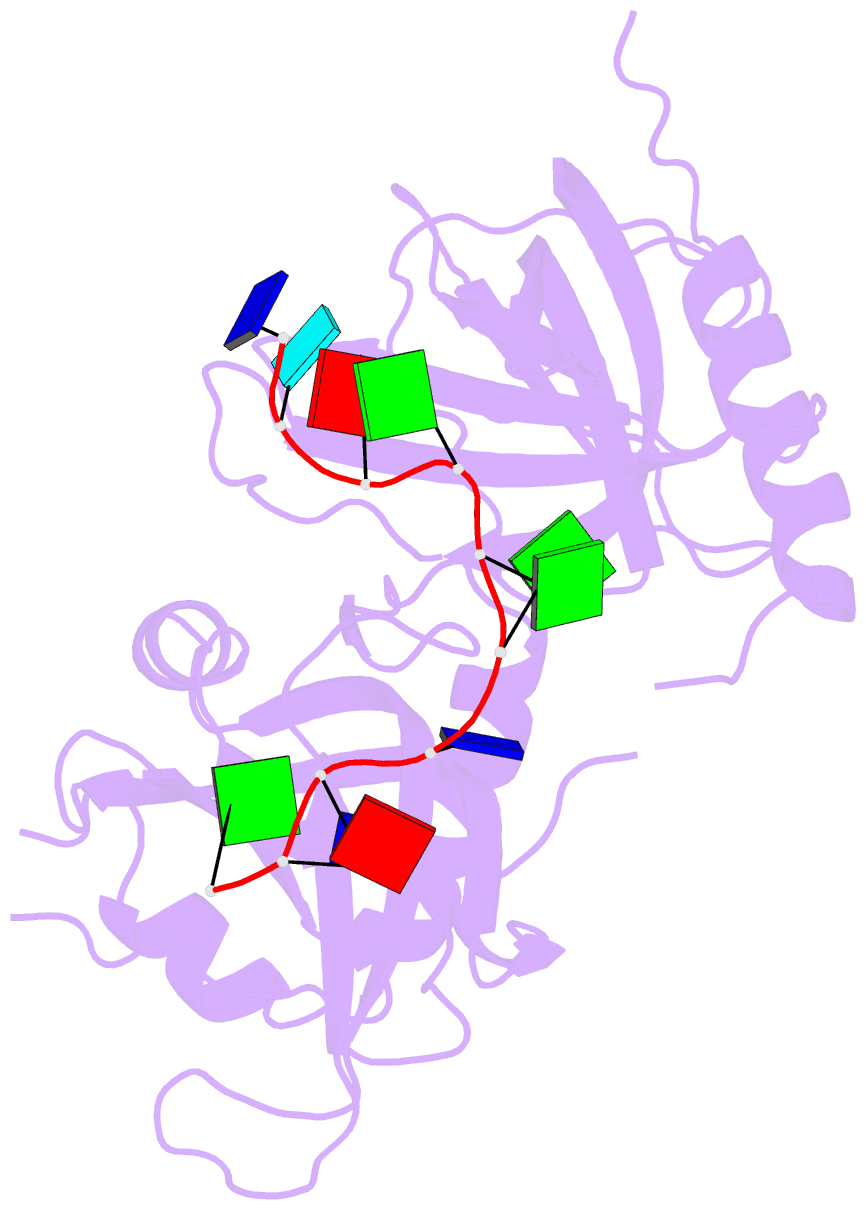
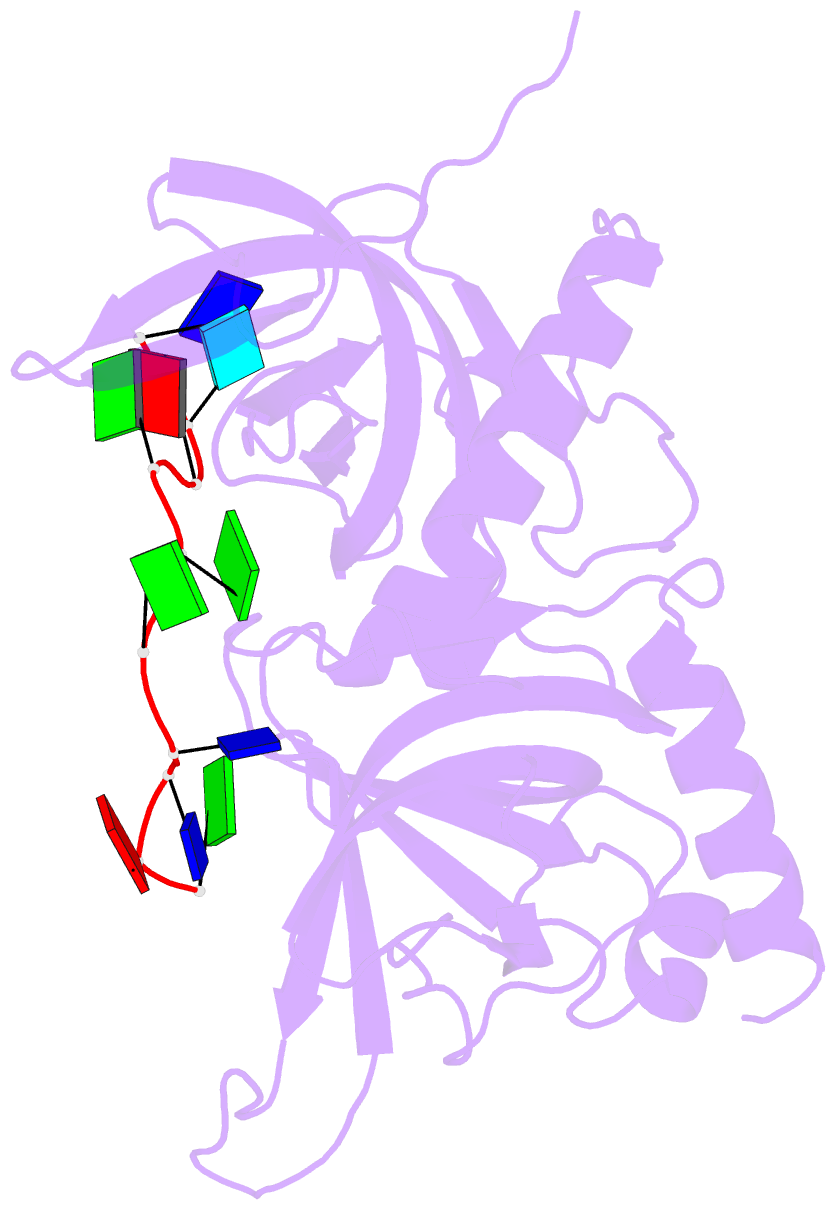
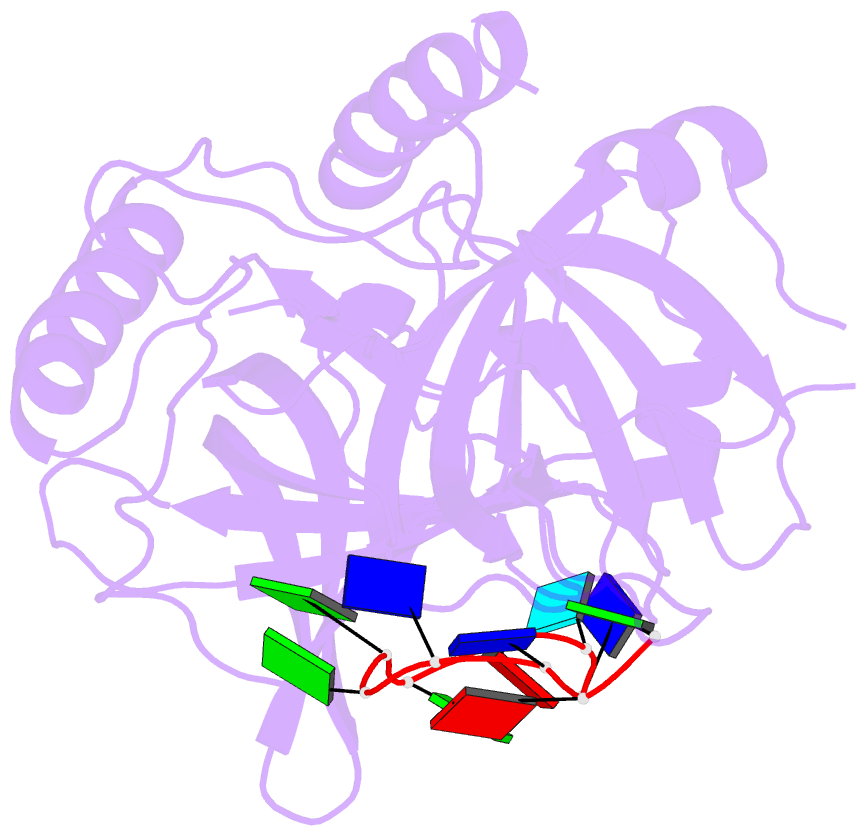
- Crystal structure of hpot1v2-dtrud(agggttag)
- Reference
- Nandakumar J, Podell ER, Cech TR (2010): "How telomeric protein POT1 avoids RNA to achieve specificity for single-stranded DNA." Proc.Natl.Acad.Sci.USA, 107, 651-656. doi: 10.1073/pnas.0911099107.
- Abstract
- The POT1-TPP1 heterodimer, the major telomere-specific single-stranded DNA-binding protein in mammalian cells, protects chromosome ends and contributes to the regulation of telomerase. The recent discovery of telomeric RNA raises the question of how POT1 faithfully binds telomeric ssDNA and avoids illicit RNA binding that could result in its depletion from telomeres. Here we show through binding studies that a single deoxythymidine in a telomeric repeat dictates the DNA versus RNA discrimination by human POT1 and mouse POT1A. We solve the crystal structure of hPOT1 bound to DNA with a ribouridine in lieu of the critical deoxythymidine and show that this substitution results in burying the 2(')-hydroxyl group in a hydrophobic region (Phe62) of POT1 in addition to eliminating favorable hydrogen-bonding interactions at the POT1-nucleic acid interface. At amino acid 62, Phe discriminates against RNA binding and Tyr allows RNA binding. We further show that TPP1 greatly augments POT1's discrimination against RNA.