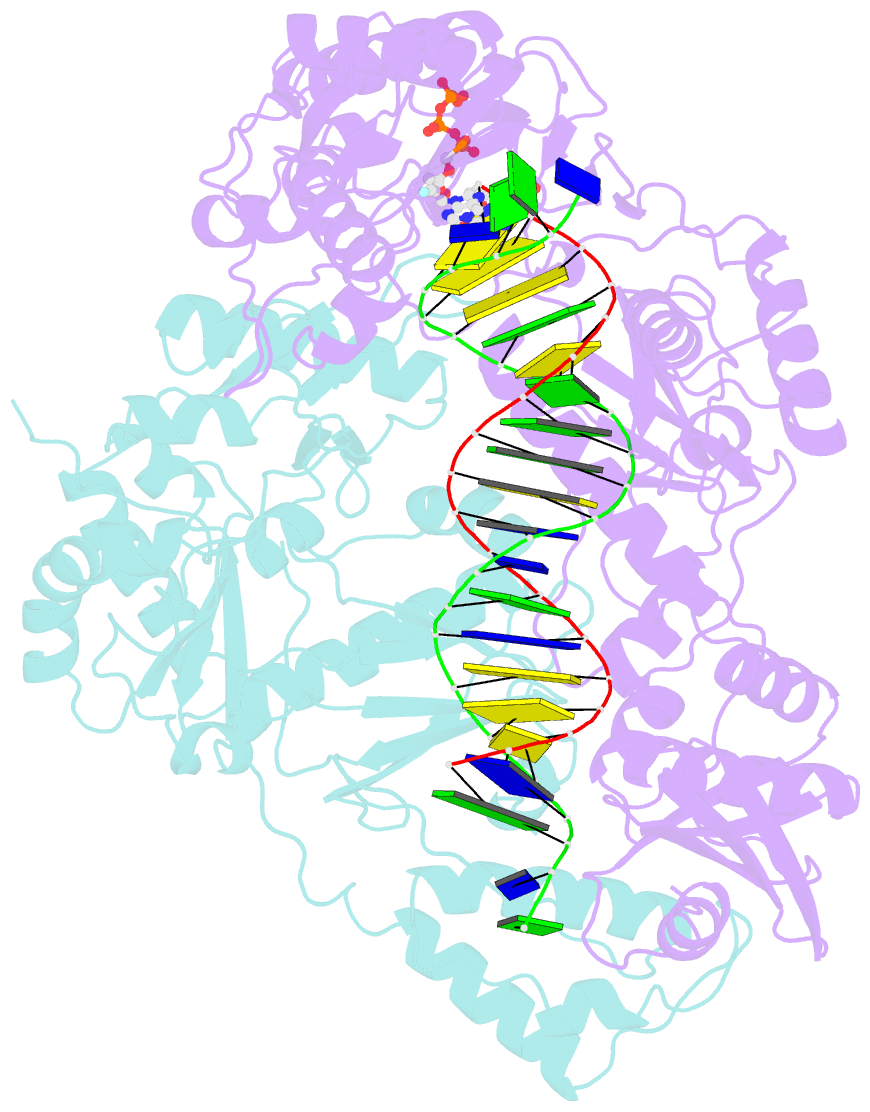
Summary information and primary citation
- PDB-id
- 3kk1; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.7 Å)
- Summary
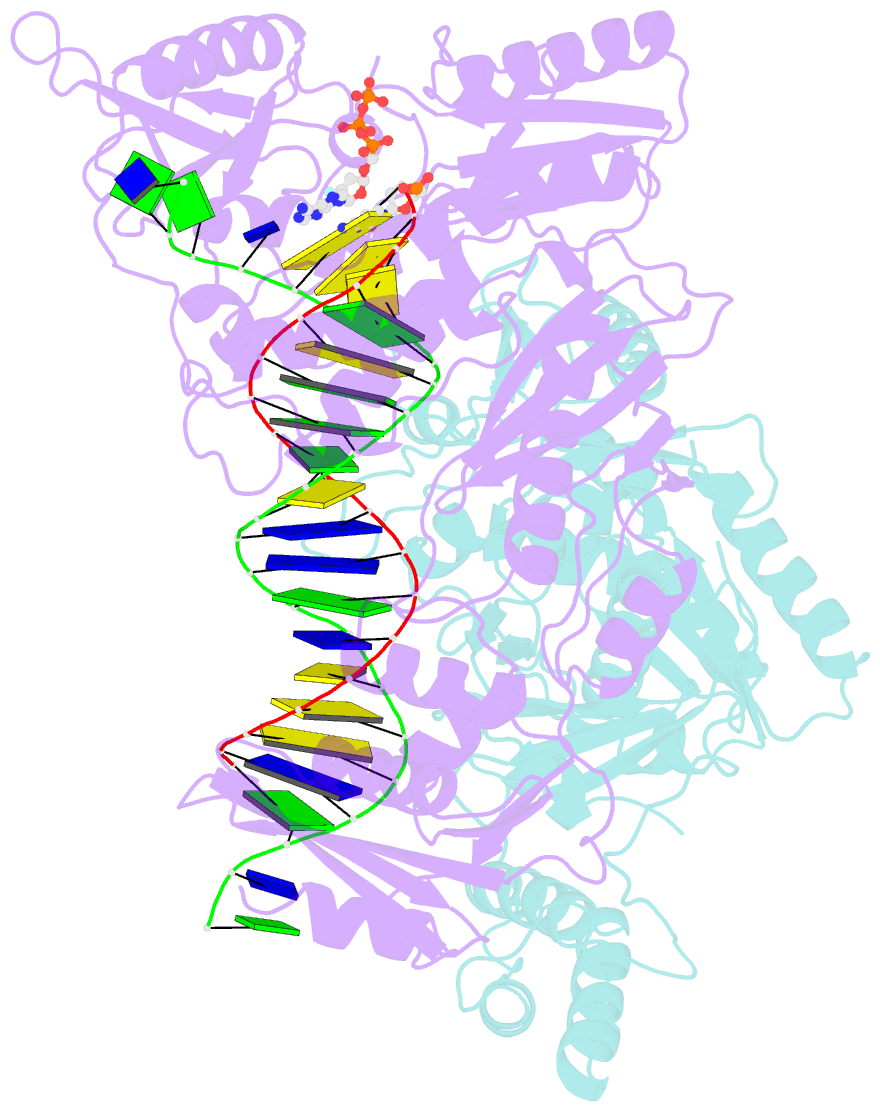
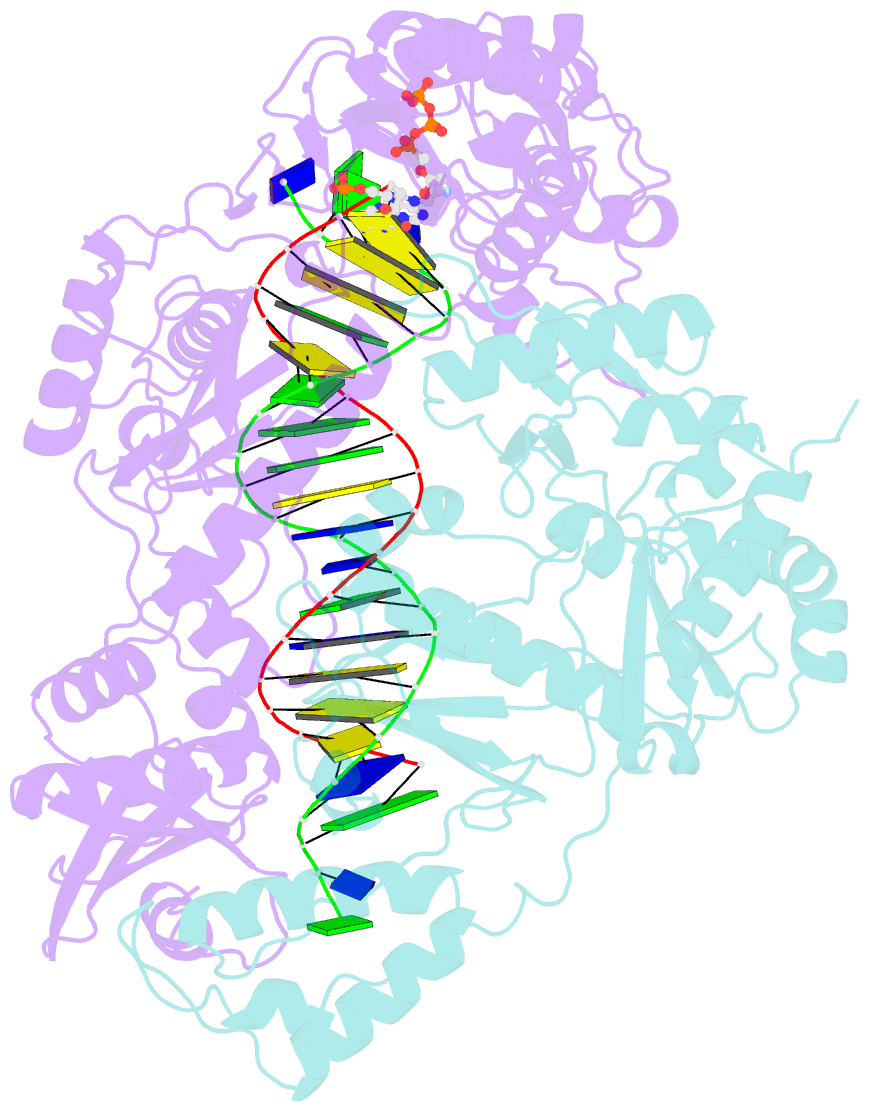
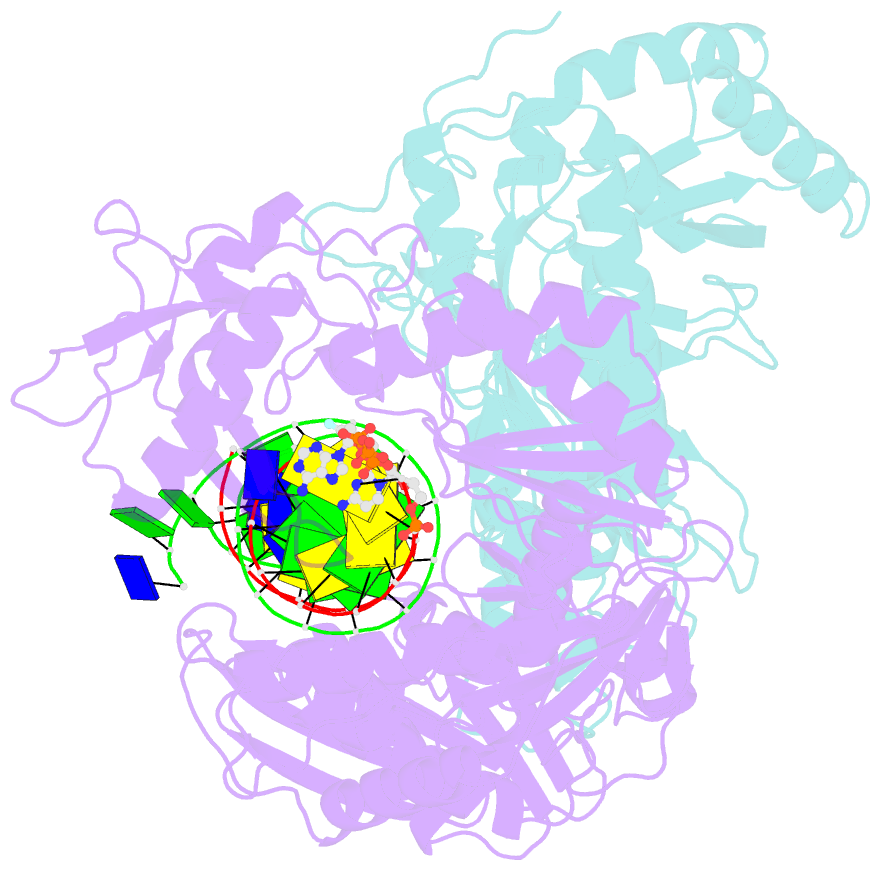
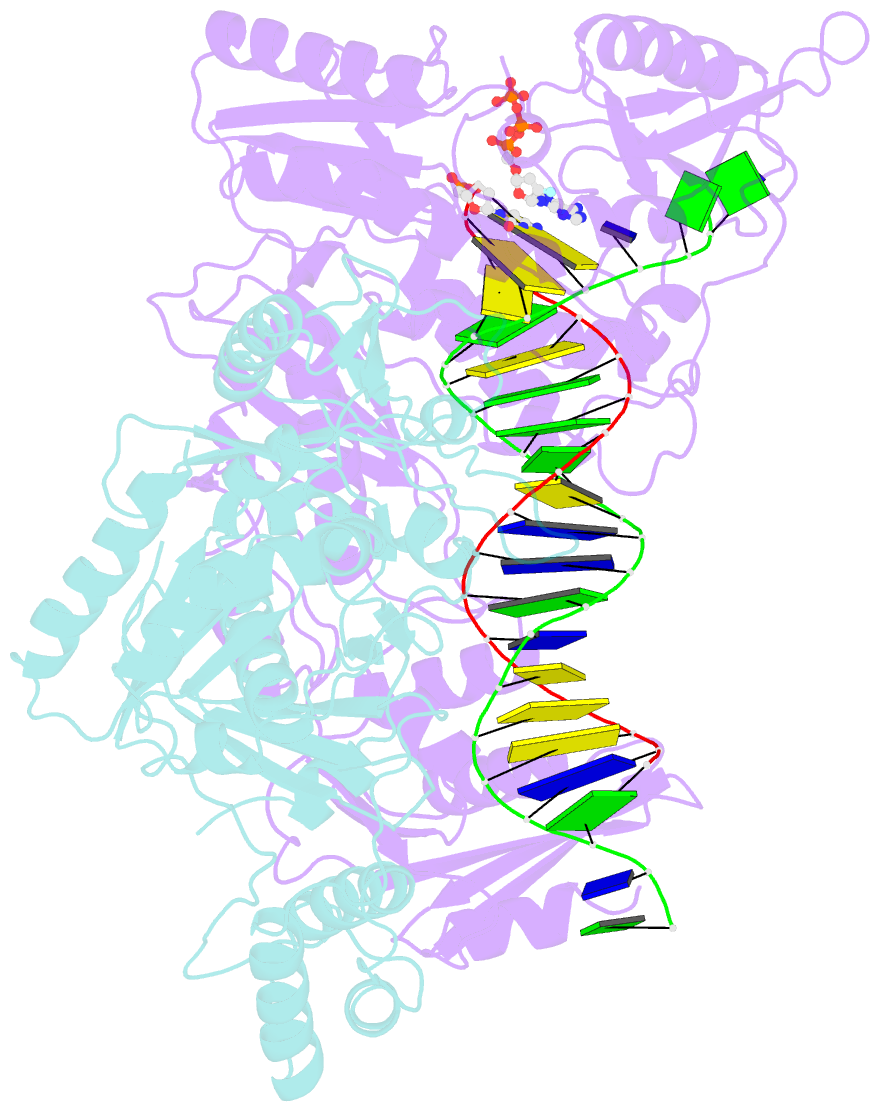
- Hiv-1 reverse transcriptase-DNA complex with nuceotide inhibitor gs-9148-diphosphate bound in nucleotide site
- Reference
- Lansdon EB, Samuel D, Lagpacan L, Brendza KM, White KL, Hung M, Liu X, Boojamra CG, Mackman RL, Cihlar T, Ray AS, McGrath ME, Swaminathan S (2010): "Visualizing the molecular interactions of a nucleotide analog, GS-9148, with HIV-1 reverse transcriptase-DNA complex." J.Mol.Biol., 397, 967-978. doi: 10.1016/j.jmb.2010.02.019.
- Abstract
- GS-9148 ([5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yloxymethyl]-phosphonic acid) is a dAMP (2'-deoxyadenosine monophosphate) analog that maintains its antiviral activity against drug-resistant HIV. Crystal structures for HIV-1 reverse transcriptase (RT) bound to double-stranded DNA, ternary complexes with either GS-9148-diphosphate or 2'-deoxyadenosine triphosphate (dATP), and a post-incorporation structure with GS-9148 translocated to the priming site were obtained to gain insight into the mechanism of RT inhibition. The binding of either GS-9148-diphosphate or dATP to the binary RT-DNA complex resulted in the fingers subdomain closing around the incoming substrate. This produced up to a 9 A shift in the tips of the fingers subdomain as it closed toward the palm and thumb subdomains. GS-9148-diphosphate shows a similar binding mode as dATP in the nucleotide-binding site. Residues whose mutations confer resistance to nucleotide/nucleoside RT inhibitors, such as M184, Y115, L74, and K65, show little to no shift in orientation whether GS-9148-diphosphate or dATP is bound. One difference observed in binding is the position of the central ring. The dihydrofuran ring of GS-9148-diphosphate interacts with the aromatic side chain of Y115 more than does the ribose ring of dATP, possibly picking up a favorable pi-pi interaction. The ability of GS-9148-diphosphate to mimic the active-site contacts of dATP may explain its effective inhibition of RT and maintained activity against resistance mutations. Interestingly, the 2'-fluoro moiety of GS-9148-diphosphate was found in close proximity to the Q151 side chain, potentially explaining the observed moderately reduced susceptibly to GS-9148 conferred by Q151M mutation.