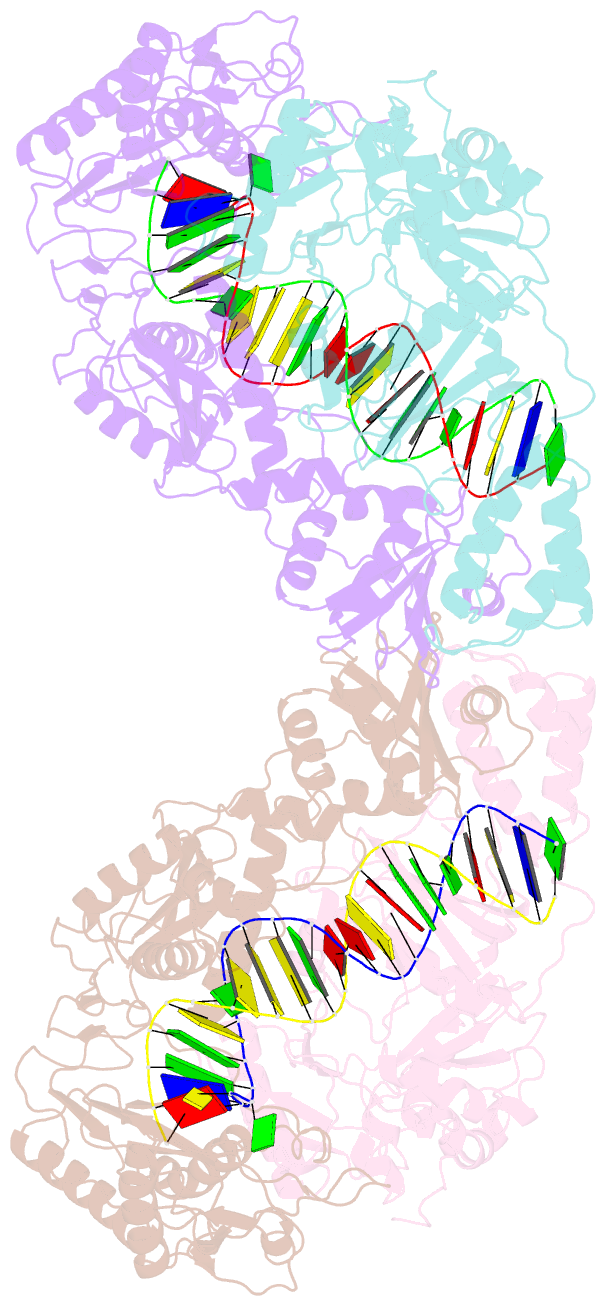
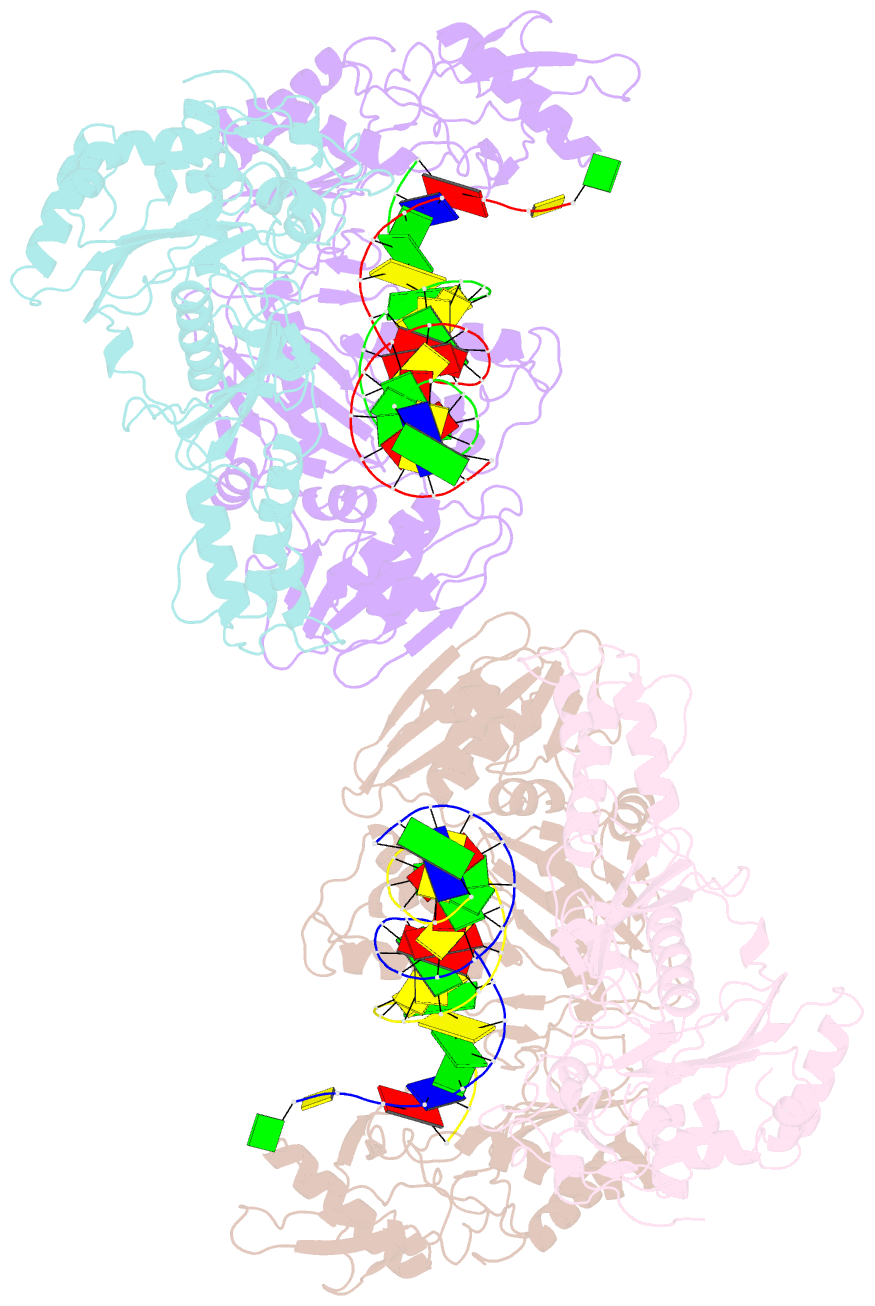
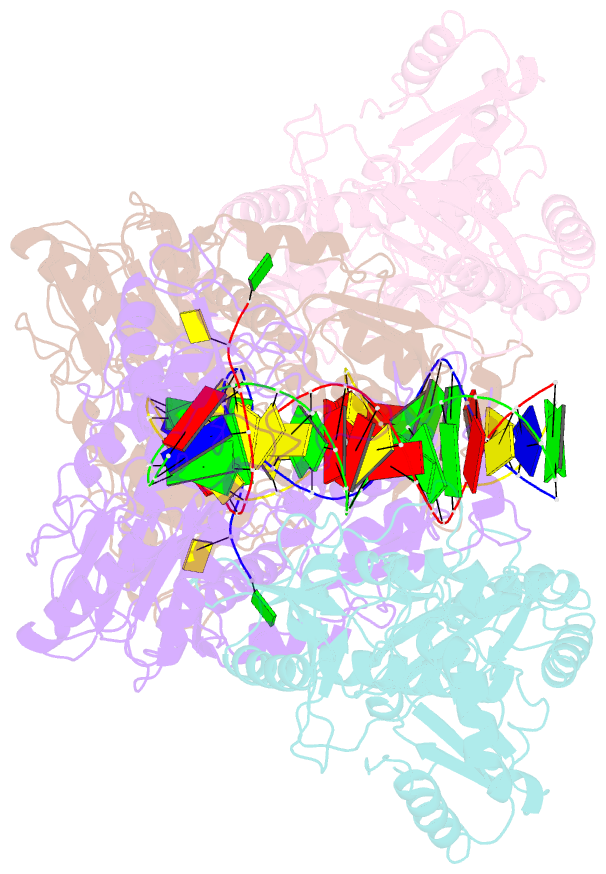
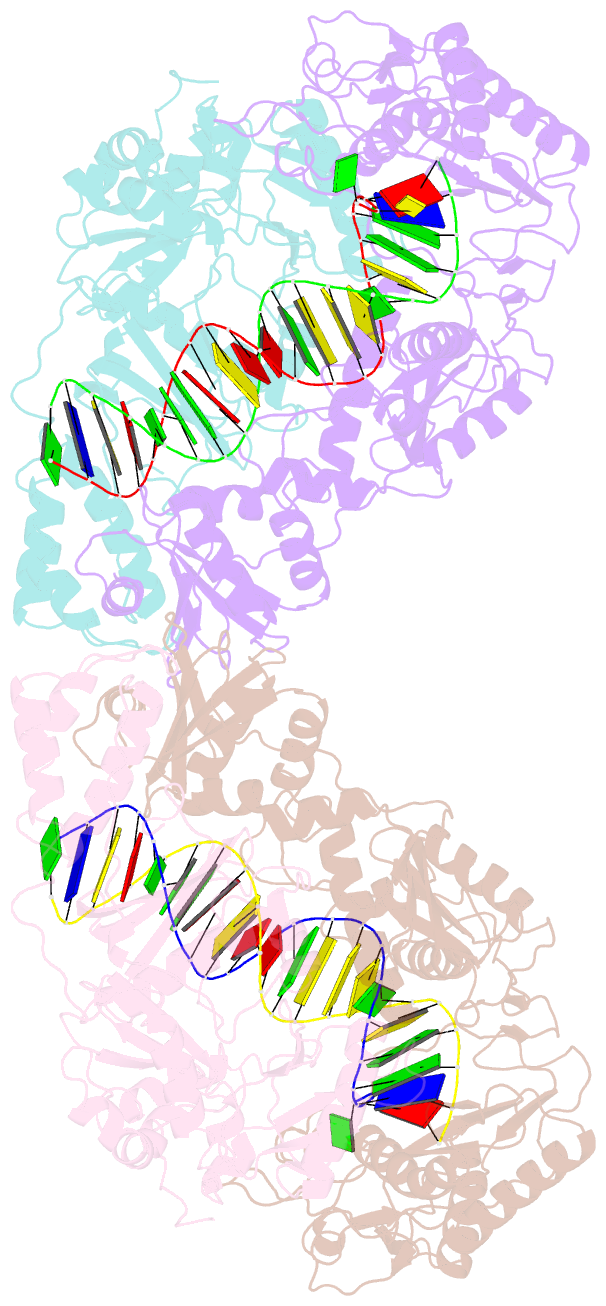
Summary information and primary citation
- PDB-id
- 3klg; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (3.65 Å)
- Summary
- Crystal structure of azt-resistant hiv-1 reverse transcriptase crosslinked to pre-translocation aztmp-terminated DNA (complex n)
- Reference
- Tu X, Das K, Han Q, Bauman JD, Clark AD, Hou X, Frenkel YV, Gaffney BL, Jones RA, Boyer PL, Hughes SH, Sarafianos SG, Arnold E (2010): "Structural basis of HIV-1 resistance to AZT by excision." Nat.Struct.Mol.Biol., 17, 1202-1209. doi: 10.1038/nsmb.1908.
- Abstract
- Human immunodeficiency virus (HIV-1) develops resistance to 3'-azido-2',3'-deoxythymidine (AZT, zidovudine) by acquiring mutations in reverse transcriptase that enhance the ATP-mediated excision of AZT monophosphate from the 3' end of the primer. The excision reaction occurs at the dNTP-binding site, uses ATP as a pyrophosphate donor, unblocks the primer terminus and allows reverse transcriptase to continue viral DNA synthesis. The excision product is AZT adenosine dinucleoside tetraphosphate (AZTppppA). We determined five crystal structures: wild-type reverse transcriptase-double-stranded DNA (RT-dsDNA)-AZTppppA; AZT-resistant (AZTr; M41L D67N K70R T215Y K219Q) RT-dsDNA-AZTppppA; AZTr RT-dsDNA terminated with AZT at dNTP- and primer-binding sites; and AZTr apo reverse transcriptase. The AMP part of AZTppppA bound differently to wild-type and AZTr reverse transcriptases, whereas the AZT triphosphate part bound the two enzymes similarly. Thus, the resistance mutations create a high-affinity ATP-binding site. The structure of the site provides an opportunity to design inhibitors of AZT-monophosphate excision.