Summary information and primary citation
- PDB-id
- 3ldy; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (1.97 Å)
- Summary
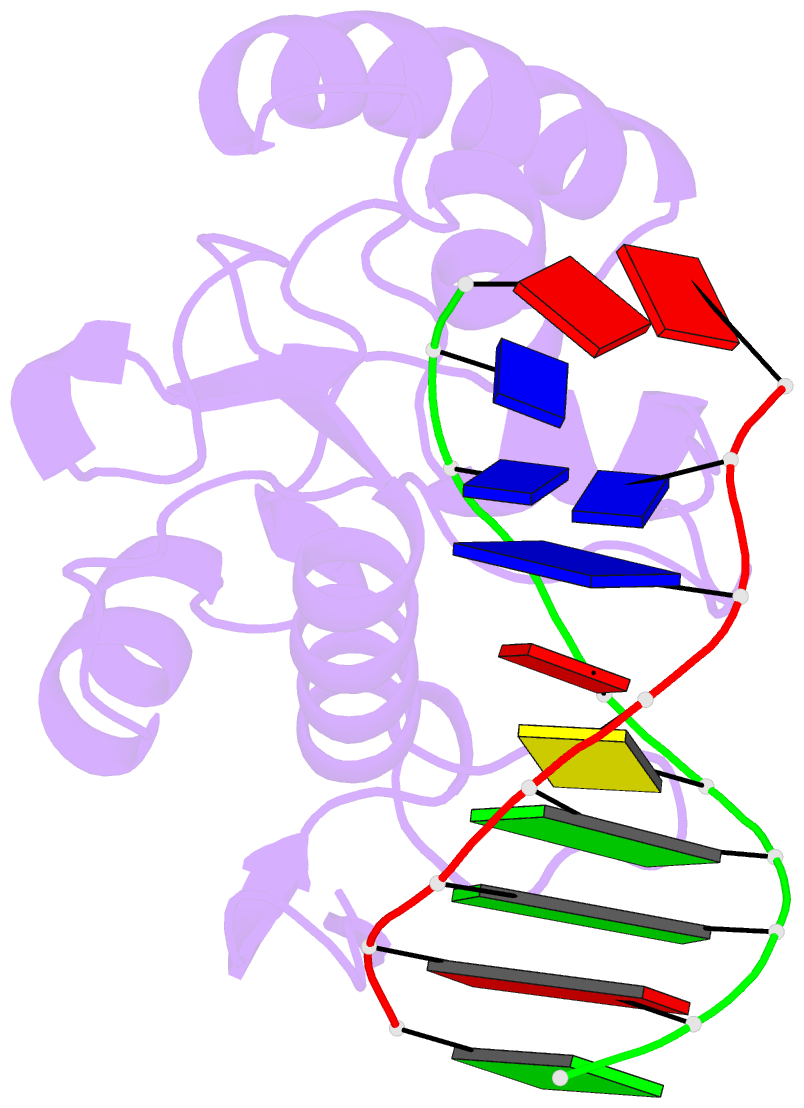
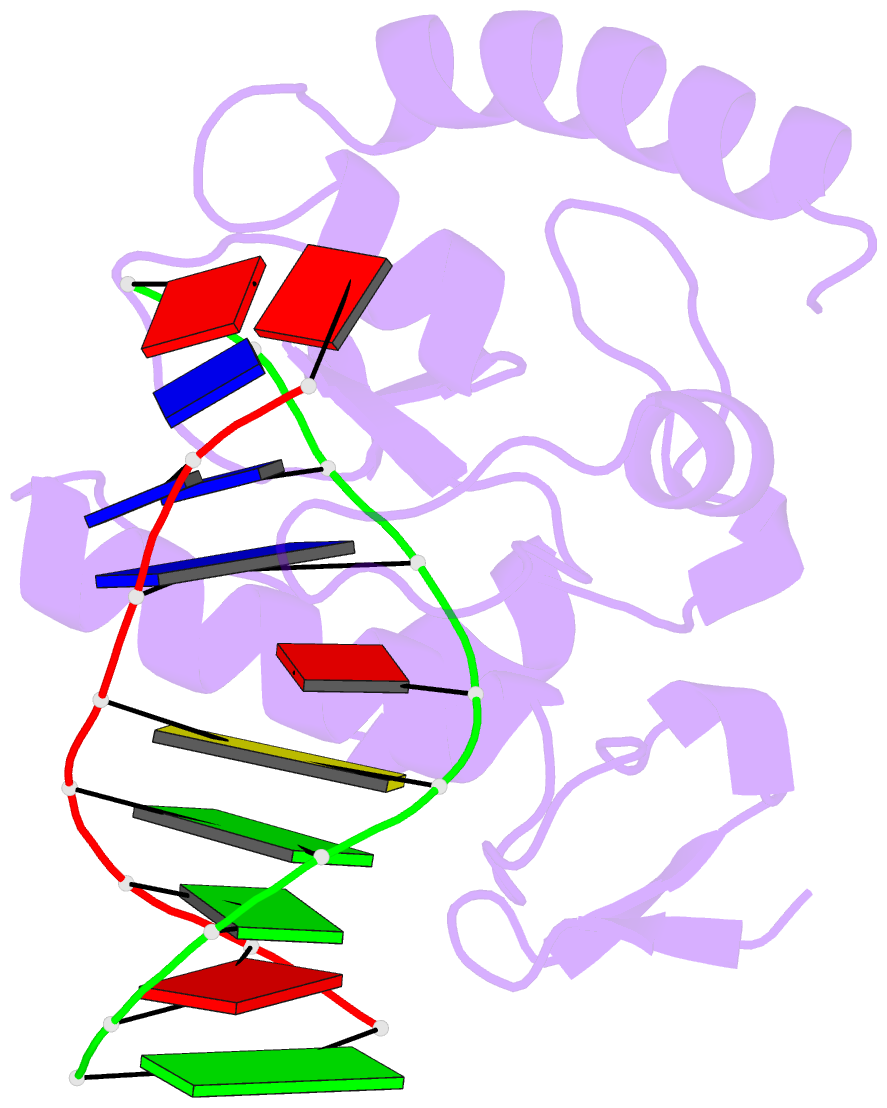
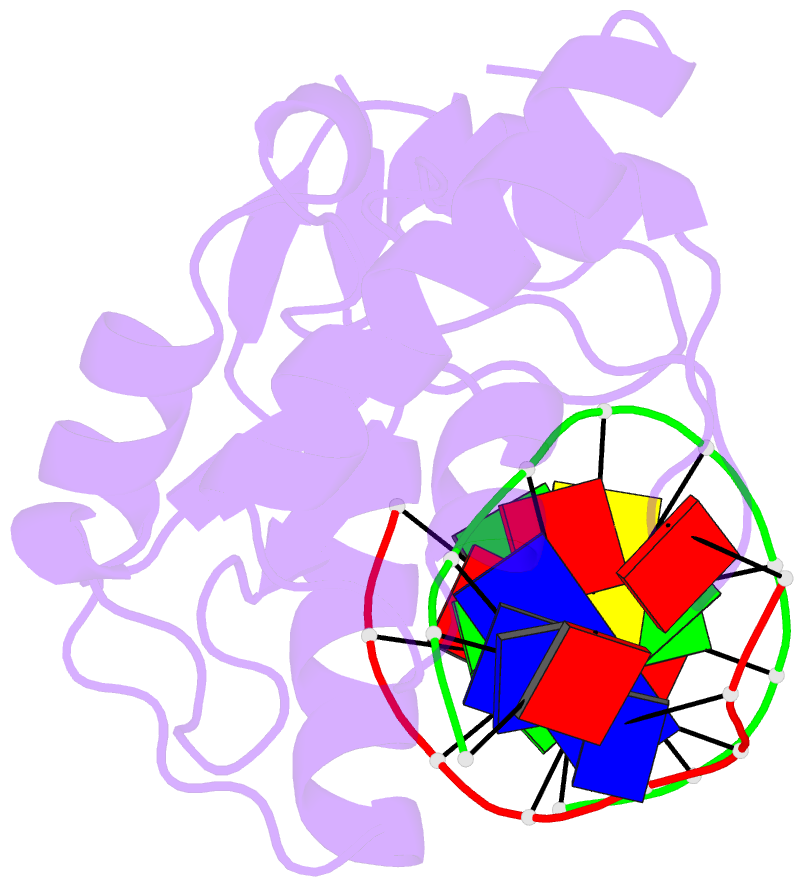
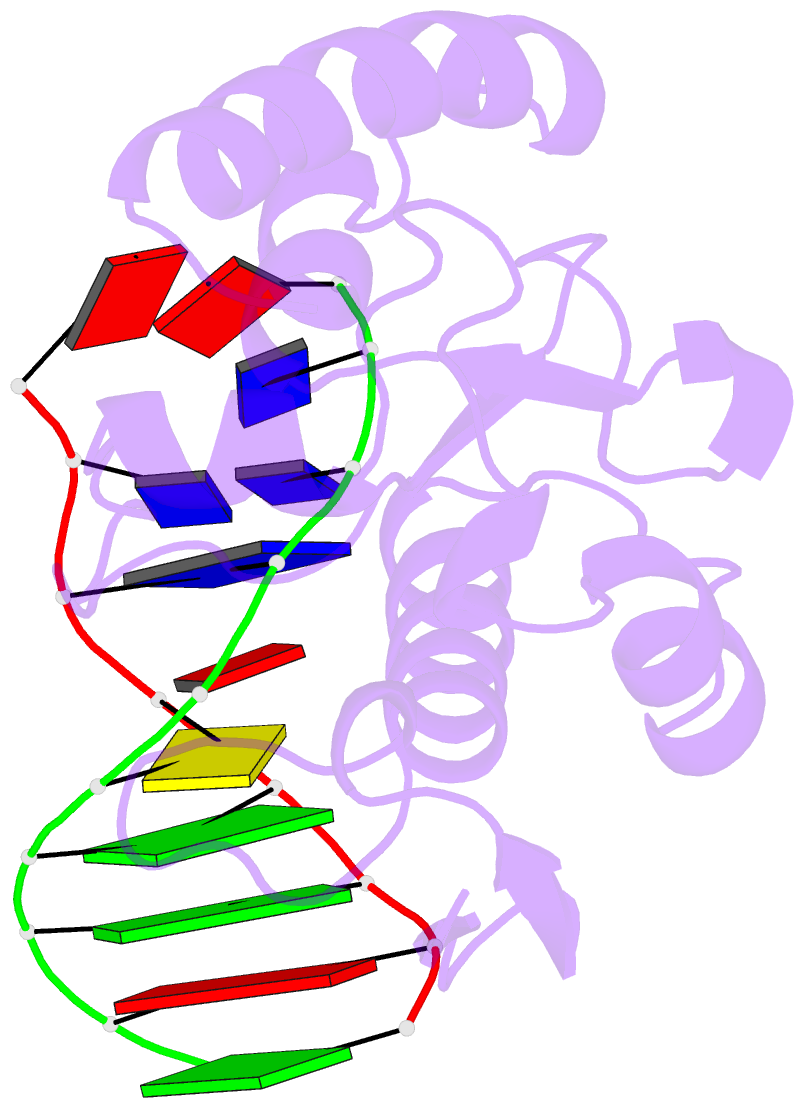
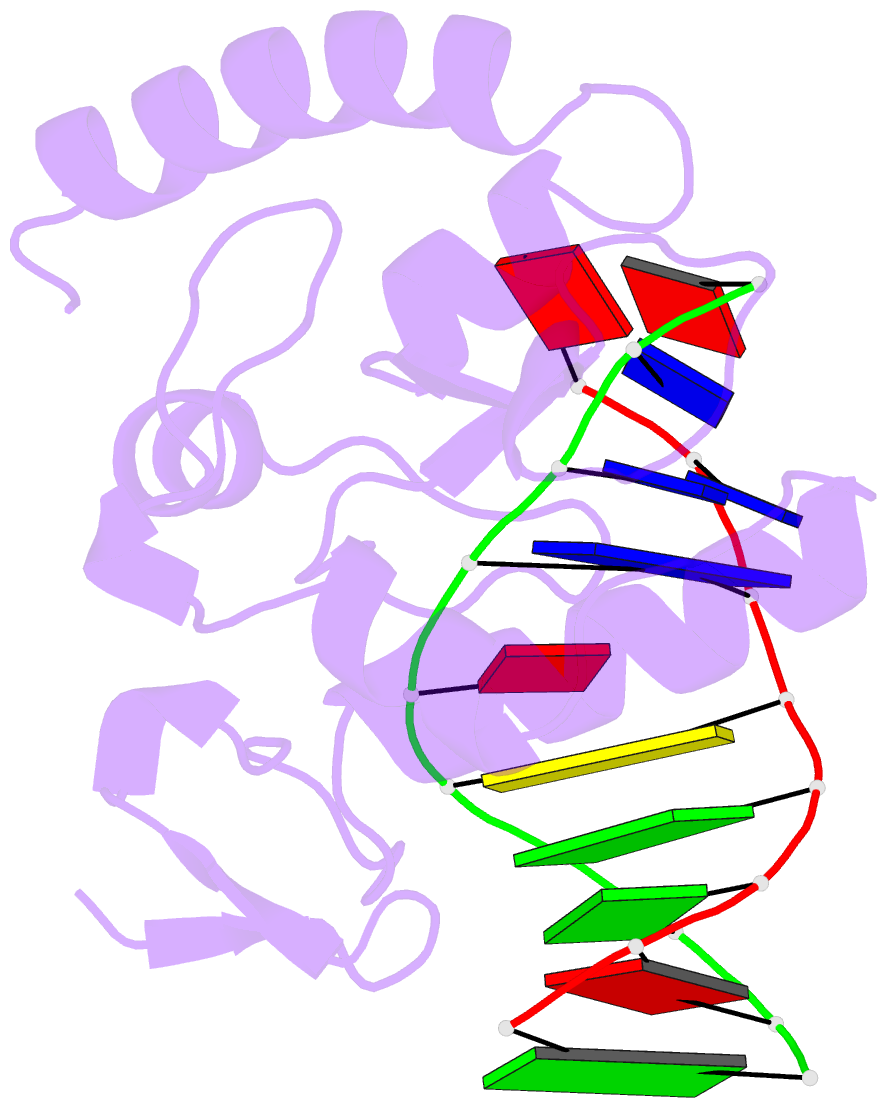
- An extraordinary mechanism of DNA perturbation exhibited by the rare-cutting hnh restriction endonuclease paci
- Reference
- Shen BW, Heiter DF, Chan SH, Wang H, Xu SY, Morgan RD, Wilson GG, Stoddard BL (2010): "Unusual target site disruption by the rare-cutting HNH restriction endonuclease PacI." Structure, 18, 734-743. doi: 10.1016/j.str.2010.03.009.
- Abstract
- The crystal structure of the rare-cutting HNH restriction endonuclease PacI in complex with its eight-base-pair target recognition sequence 5'-TTAATTAA-3' has been determined to 1.9 A resolution. The enzyme forms an extended homodimer, with each subunit containing two zinc-bound motifs surrounding a betabetaalpha-metal catalytic site. The latter is unusual in that a tyrosine residue likely initiates strand cleavage. PacI dramatically distorts its target sequence from Watson-Crick duplex DNA base pairing, with every base separated from its original partner. Two bases on each strand are unpaired, four are engaged in noncanonical A:A and T:T base pairs, and the remaining two bases are matched with new Watson-Crick partners. This represents a highly unusual DNA binding mechanism for a restriction endonuclease, and implies that initial recognition of the target site might involve significantly different contacts from those visualized in the DNA-bound cocrystal structures.