Summary information and primary citation
- PDB-id
- 3mlo; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (3.01 Å)
- Summary
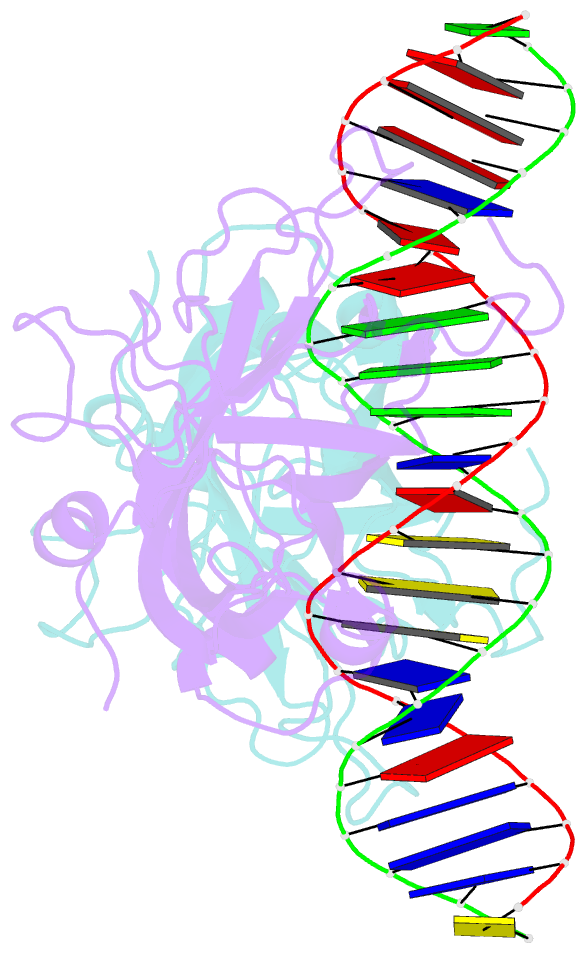
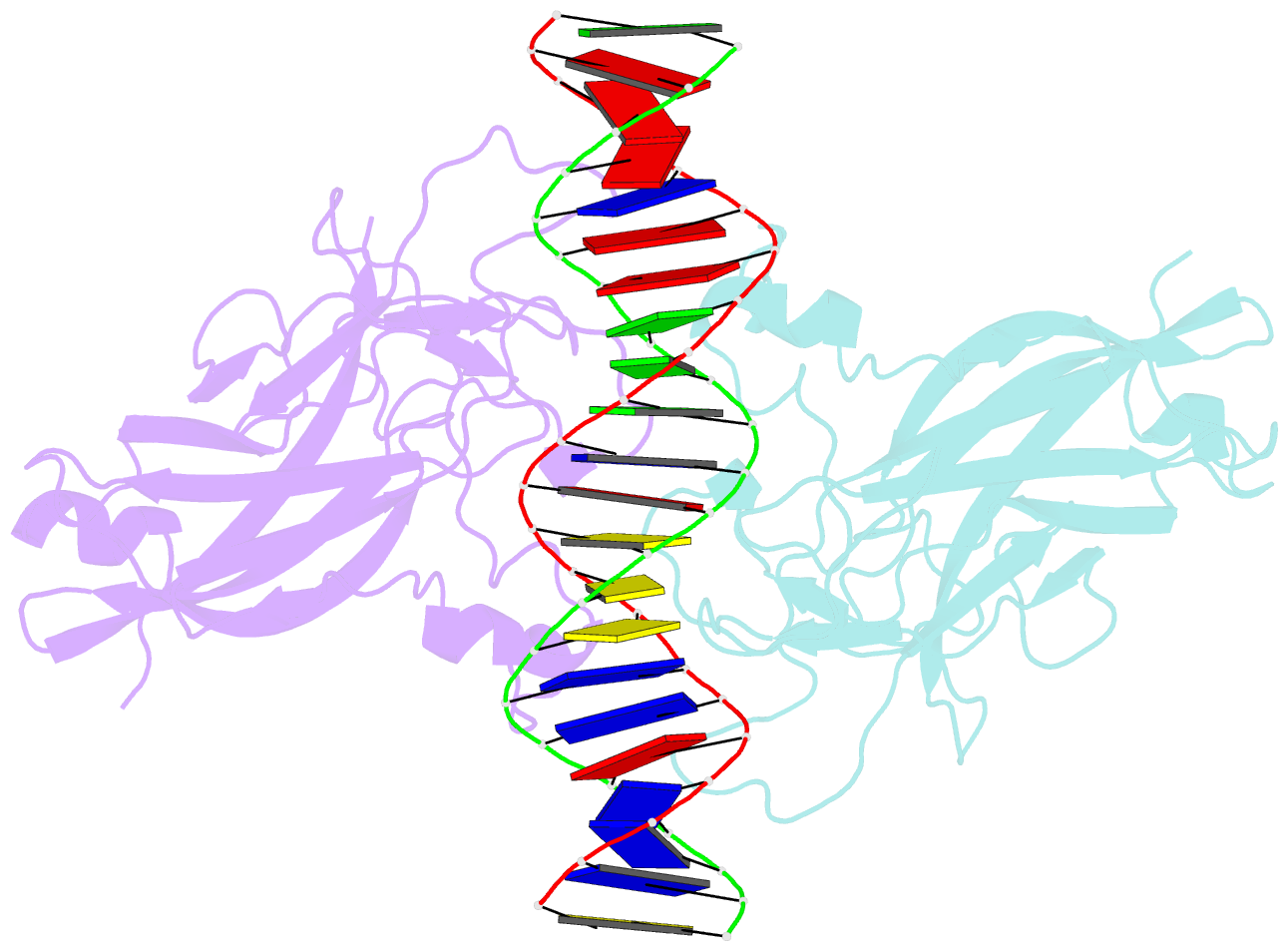
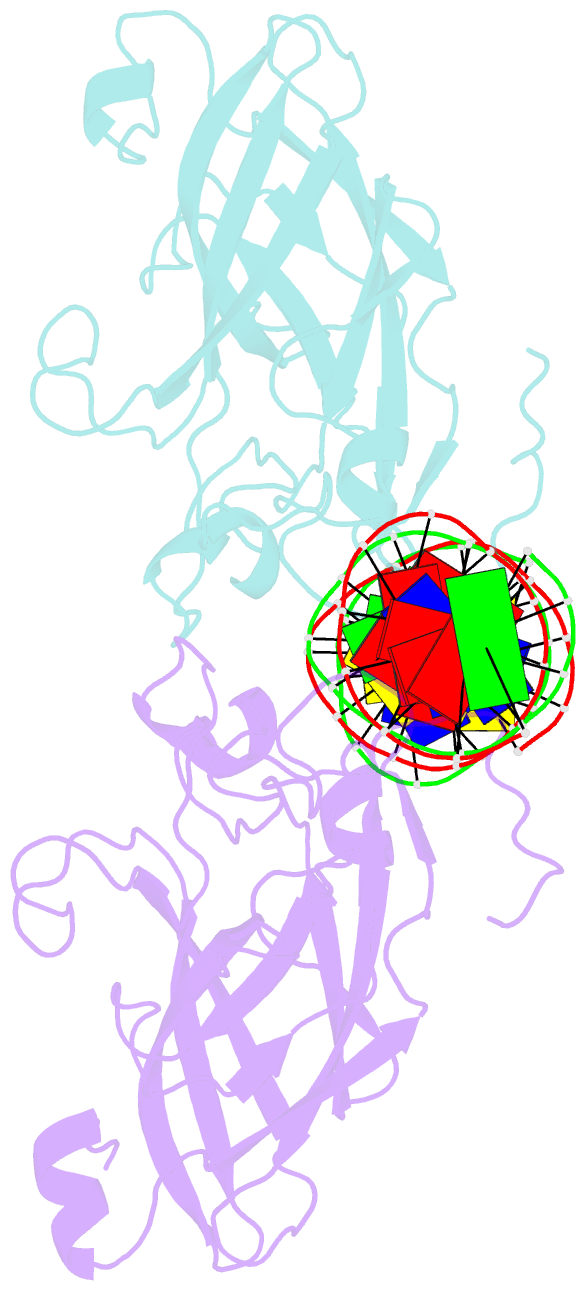
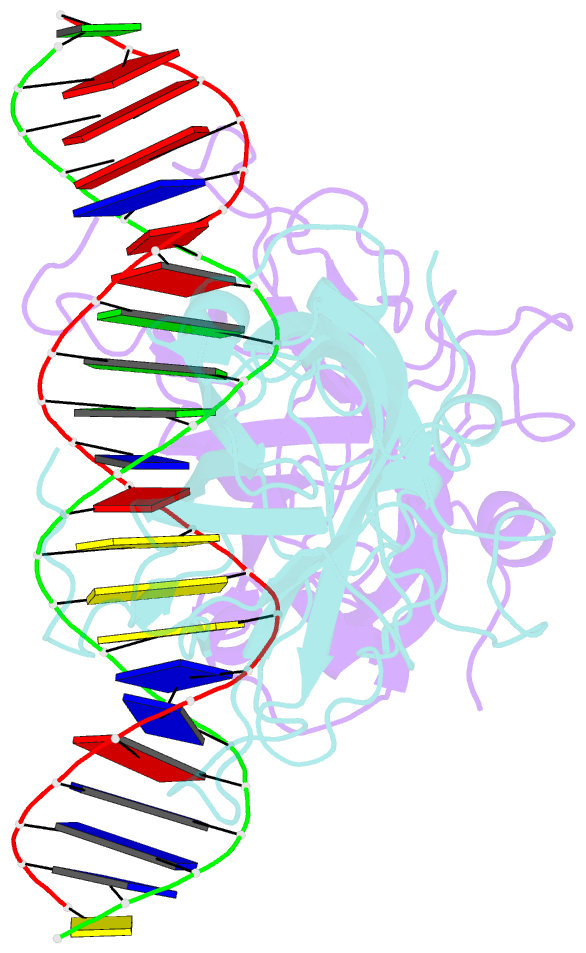
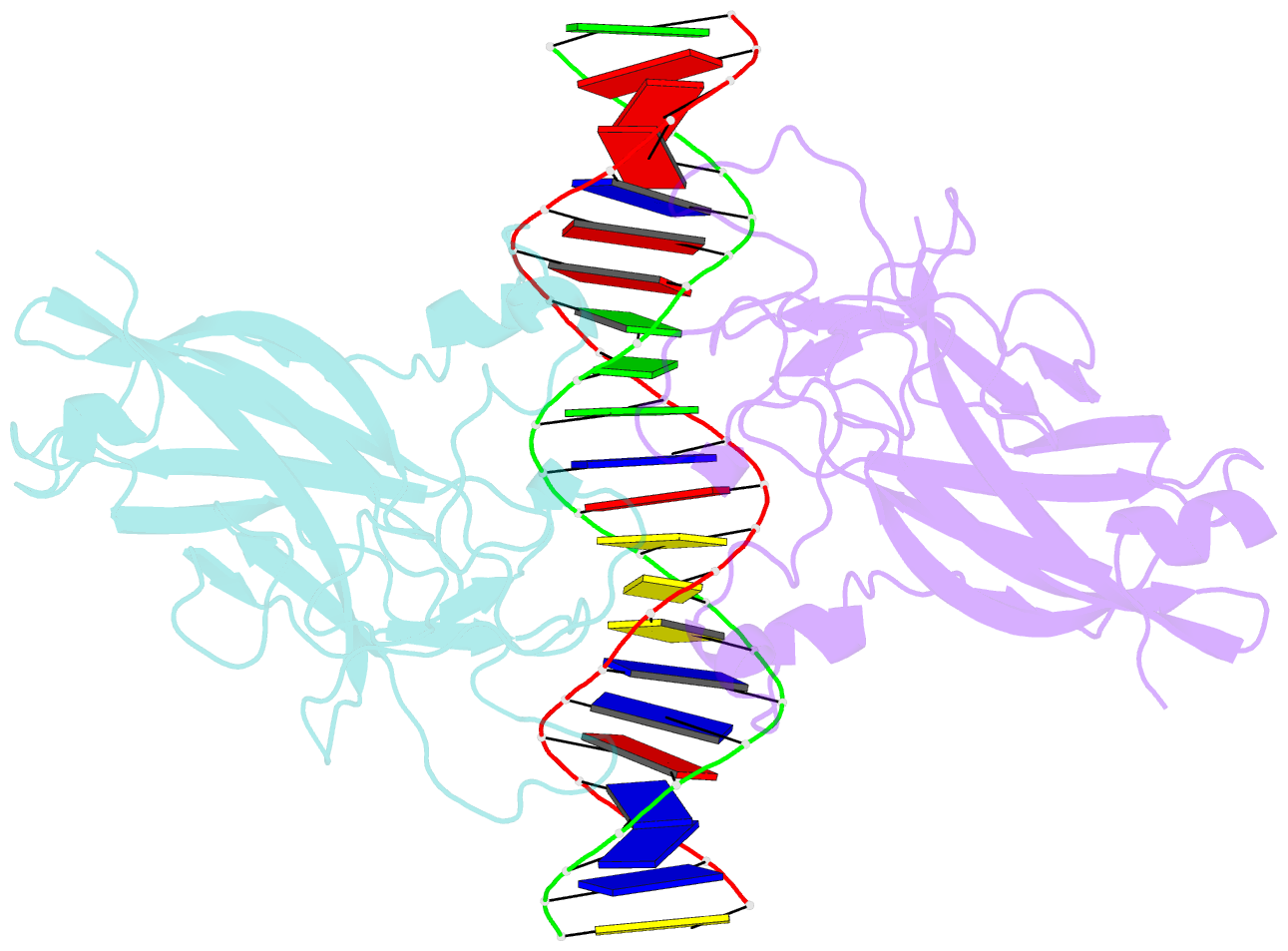
- DNA binding domain of early b-cell factor 1 (ebf1) bound to DNA (crystal form i)
- Reference
- Treiber N, Treiber T, Zocher G, Grosschedl R (2010): "Structure of an Ebf1:DNA complex reveals unusual DNA recognition and structural homology with Rel proteins." Genes Dev., 24, 2270-2275. doi: 10.1101/gad.1976610.
- Abstract
- Early B-cell factor 1 (Ebf1) is a key transcriptional determinant of B-lymphocyte differentiation whose DNA-binding domain has no sequence similarity to other transcription factor families. Here we report the crystal structure of an Ebf1 dimer bound to its palindromic recognition site. The DNA-binding domain adopts a pseudoimmunoglobulin-like fold with novel topology, but is structurally similar to the Rel homology domains of NFAT and NF-κB. Ebf1 contacts the DNA with two loop-based modules and a unique Zn coordination motif whereby each Ebf1 monomer interacts with both palindromic half-sites. This unusual mode of DNA recognition generates an extended contact area that may be crucial for the function of Ebf1 in chromatin.