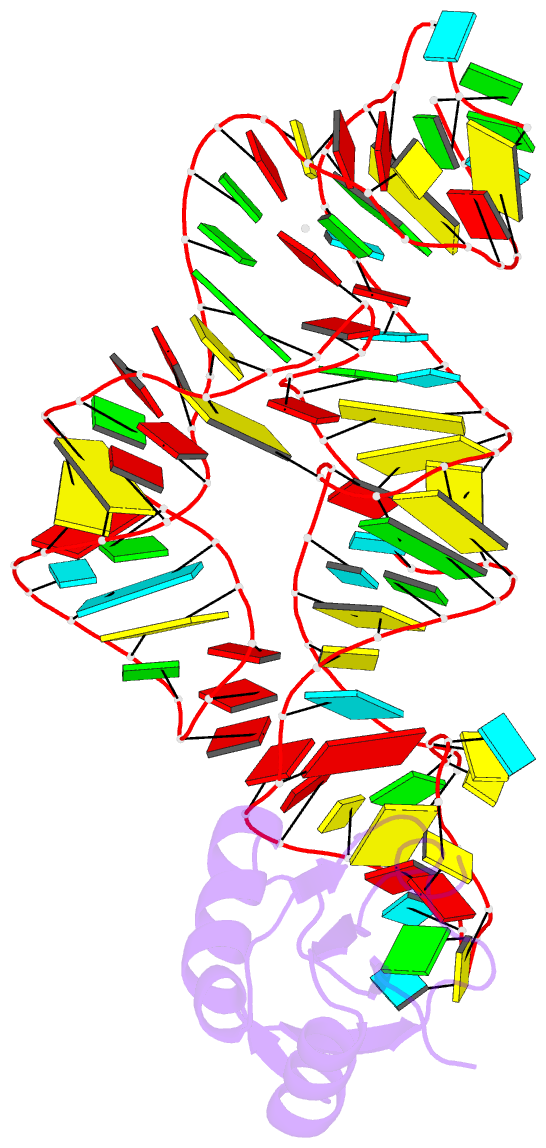
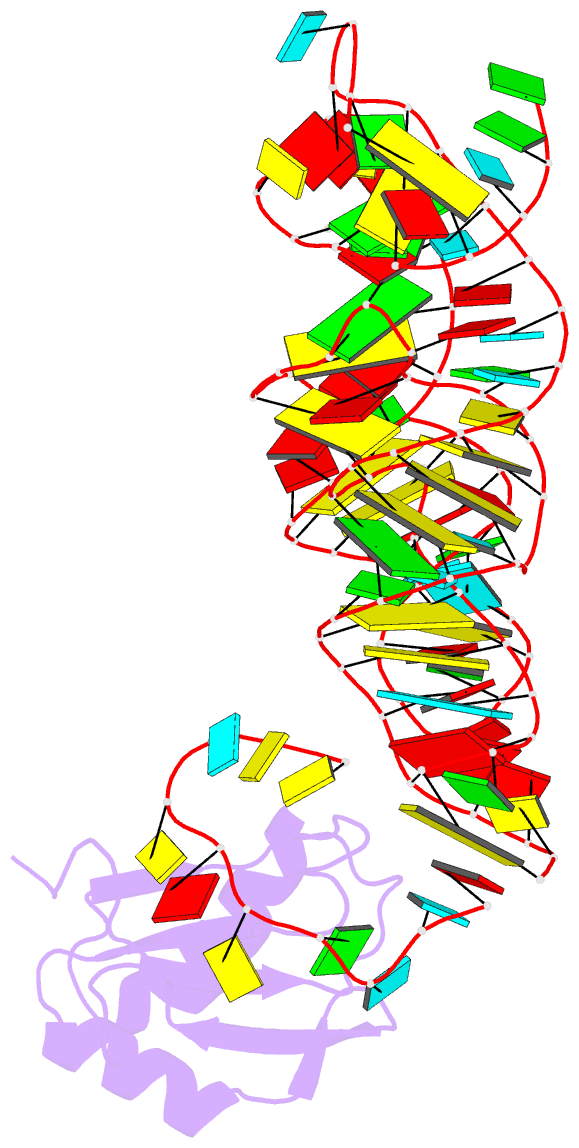
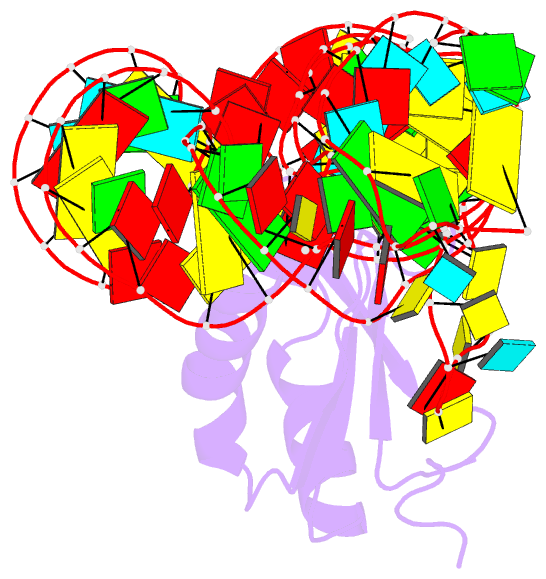
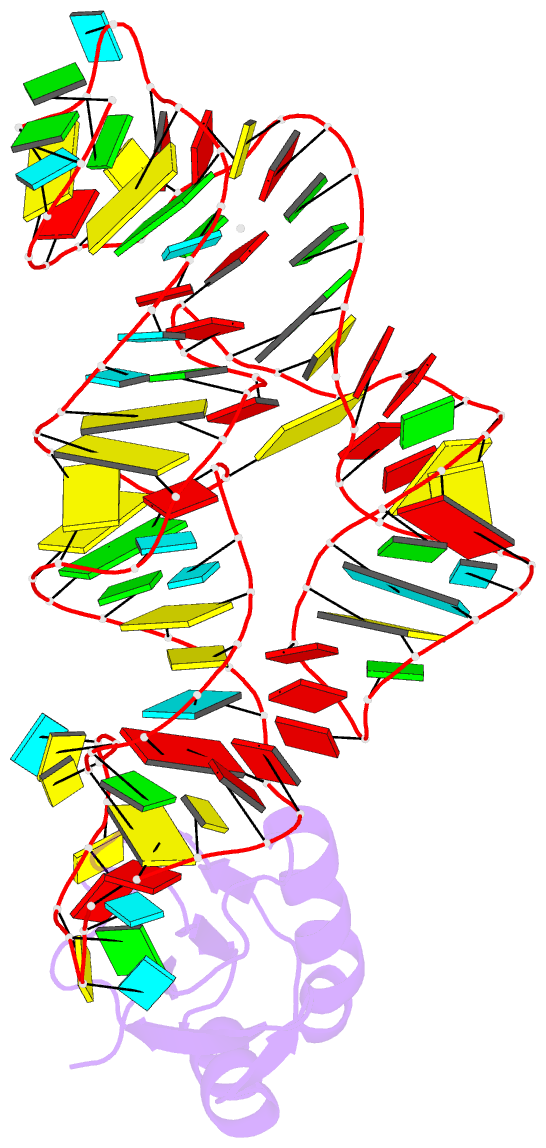
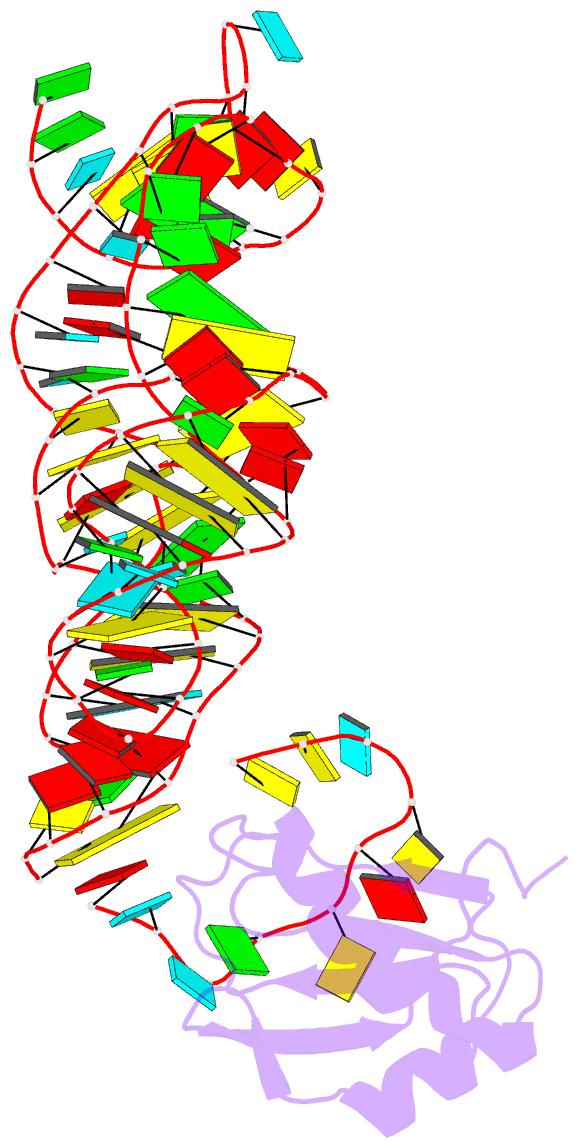
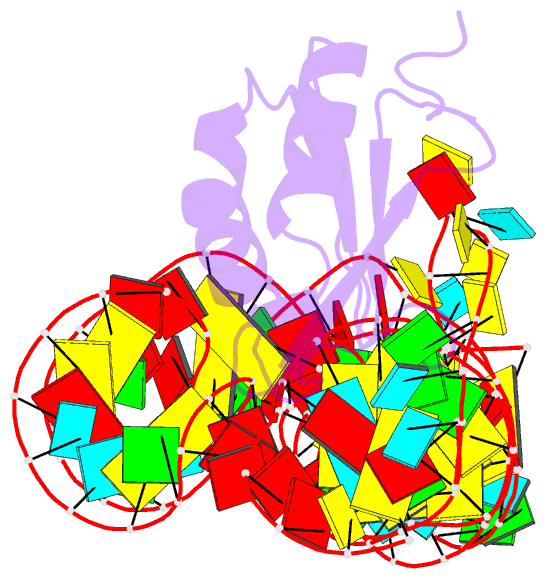
Summary information and primary citation
- PDB-id
- 3mur; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (3.0 Å)
- Summary
- Crystal structure of the c92u mutant c-di-gmp riboswith bound to c-di-gmp
- Reference
- Smith KD, Lipchock SV, Livingston AL, Shanahan CA, Strobel SA (2010): "Structural and biochemical determinants of ligand binding by the c-di-GMP riboswitch ." Biochemistry, 49, 7351-7359. doi: 10.1021/bi100671e.
- Abstract
- The bacterial second messenger c-di-GMP is used in many species to control essential processes that allow the organism to adapt to its environment. The c-di-GMP riboswitch (GEMM) is an important downstream target in this signaling pathway and alters gene expression in response to changing concentrations of c-di-GMP. The riboswitch selectively recognizes its second messenger ligand primarily through contacts with two critical nucleotides. However, these two nucleotides are not the most highly conserved residues within the riboswitch sequence. Instead, nucleotides that stack with c-di-GMP and that form tertiary RNA contacts are the most invariant. Biochemical and structural evidence reveals that the most common natural variants are able to make alternative pairing interactions with both guanine bases of the ligand. Additionally, a high-resolution (2.3 A) crystal structure of the native complex reveals that a single metal coordinates the c-di-GMP backbone. Evidence is also provided that after transcription of the first nucleotide on the 3'-side of the P1 helix, which is predicted to be the molecular switch, the aptamer is functional for ligand binding. Although large energetic effects occur when several residues in the RNA are altered, mutations at the most conserved positions, rather than at positions that base pair with c-di-GMP, have the most detrimental effects on binding. Many mutants retain sufficient c-di-GMP affinity for the RNA to remain biologically relevant, which suggests that this motif is quite resilient to mutation.