Summary information and primary citation
- PDB-id
- 3nm9; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-DNA
- Method
- X-ray (2.85 Å)
- Summary
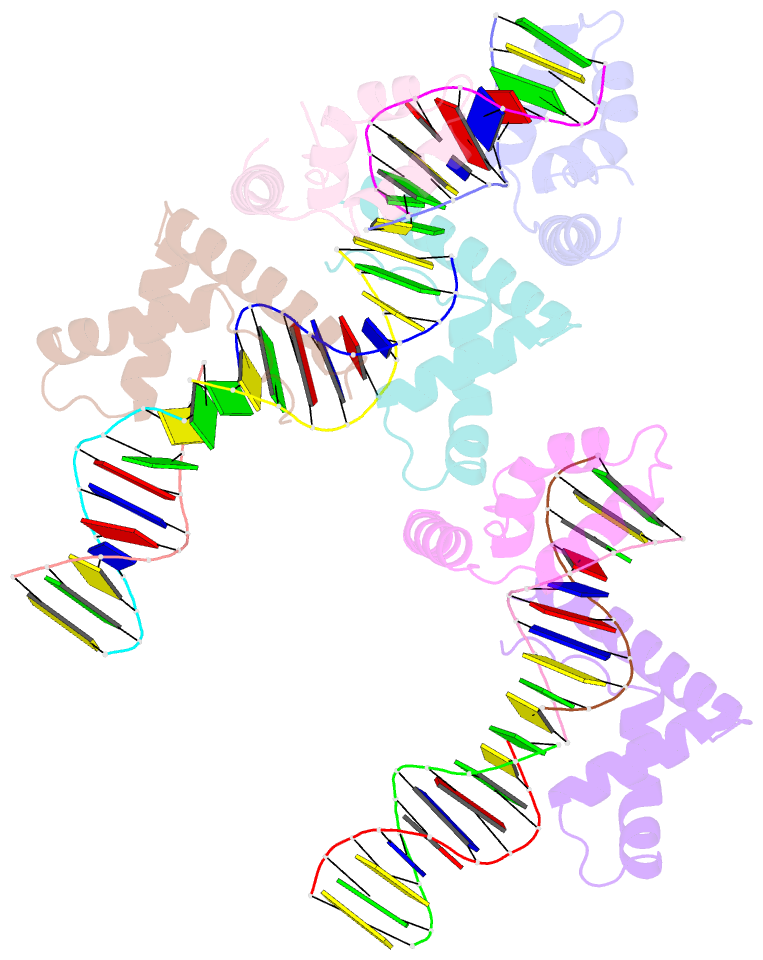
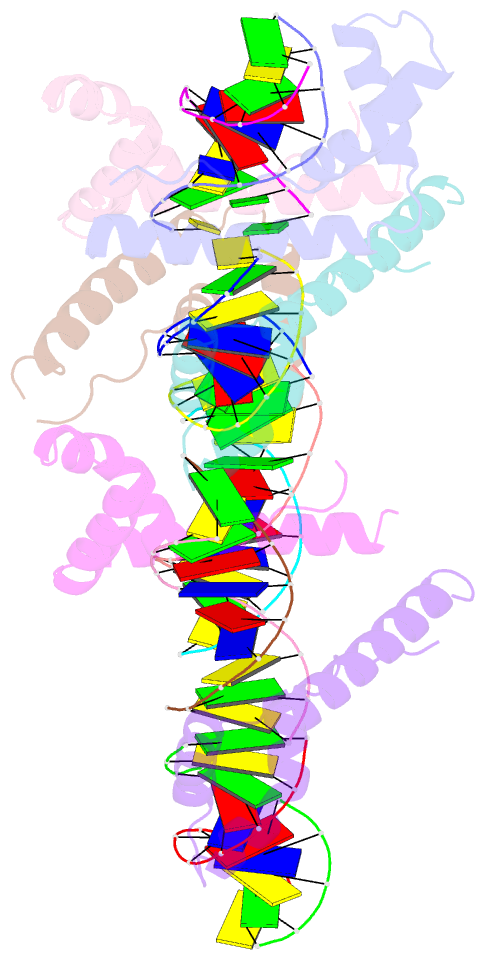
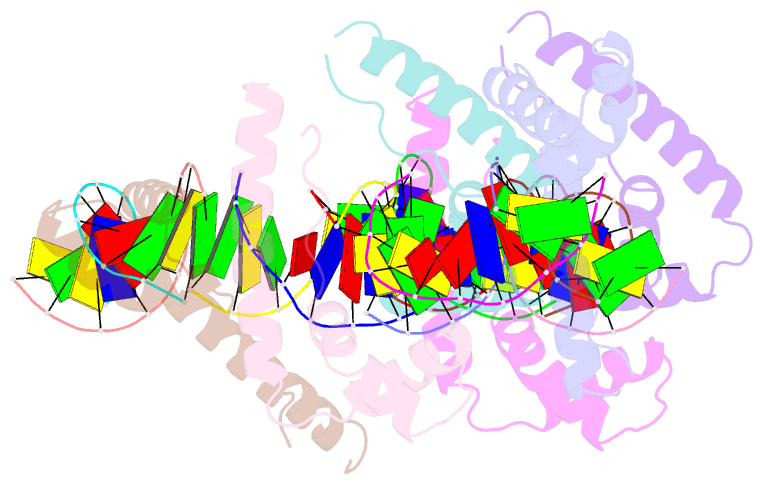
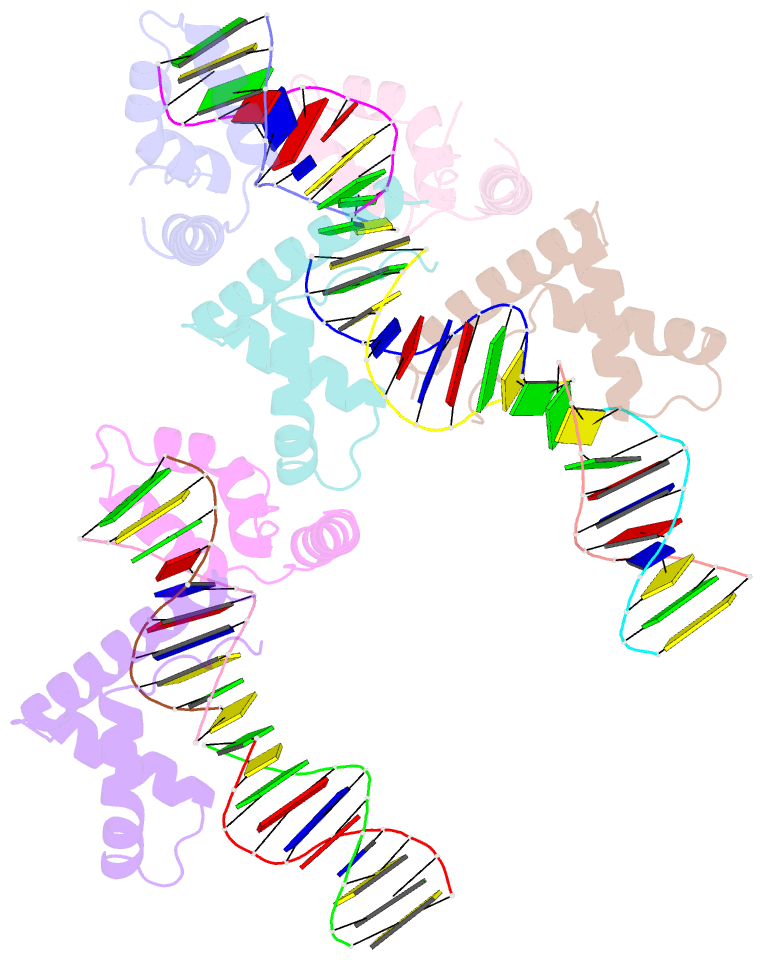
- Hmgd(m13a)-DNA complex
- Reference
- Churchill ME, Klass J, Zoetewey DL (2010): "Structural analysis of HMGD-DNA complexes reveals influence of intercalation on sequence selectivity and DNA bending." J.Mol.Biol., 403, 88-102. doi: 10.1016/j.jmb.2010.08.031.
- Abstract
- The ubiquitous, eukaryotic, high-mobility group box (HMGB) chromosomal proteins promote many chromatin-mediated cellular activities through their non-sequence-specific binding and bending of DNA. Minor-groove DNA binding by the HMG box results in substantial DNA bending toward the major groove owing to electrostatic interactions, shape complementarity, and DNA intercalation that occurs at two sites. Here, the structures of the complexes formed with DNA by a partially DNA intercalation-deficient mutant of Drosophila melanogaster HMGD have been determined by X-ray crystallography at a resolution of 2.85 Å. The six proteins and 50 bp of DNA in the crystal structure revealed a variety of bound conformations. All of the proteins bound in the minor groove, bridging DNA molecules, presumably because these DNA regions are easily deformed. The loss of the primary site of DNA intercalation decreased overall DNA bending and shape complementarity. However, DNA bending at the secondary site of intercalation was retained and most protein-DNA contacts were preserved. The mode of binding resembles the HMGB1 box A-cisplatin-DNA complex, which also lacks a primary intercalating residue. This study provides new insights into the binding mechanisms used by HMG boxes to recognize varied DNA structures and sequences as well as modulate DNA structure and DNA bending.