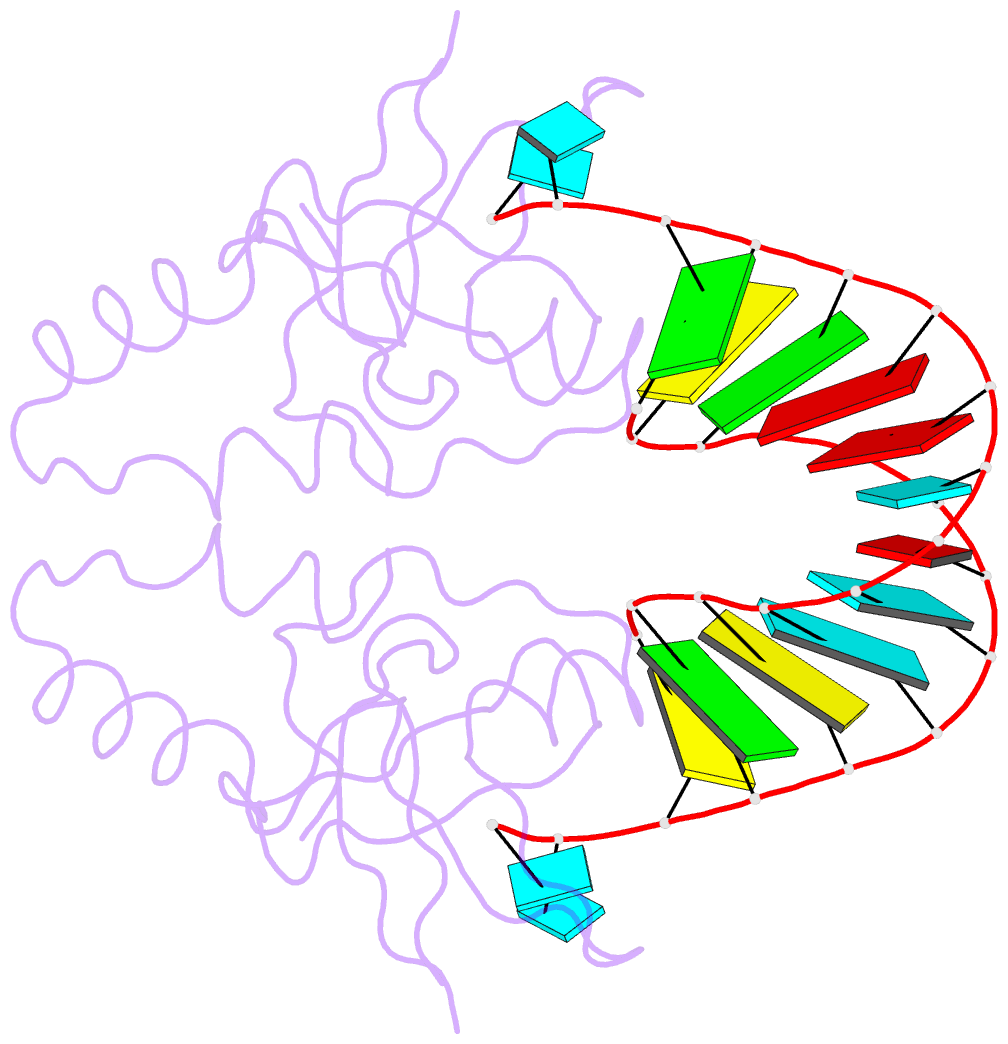
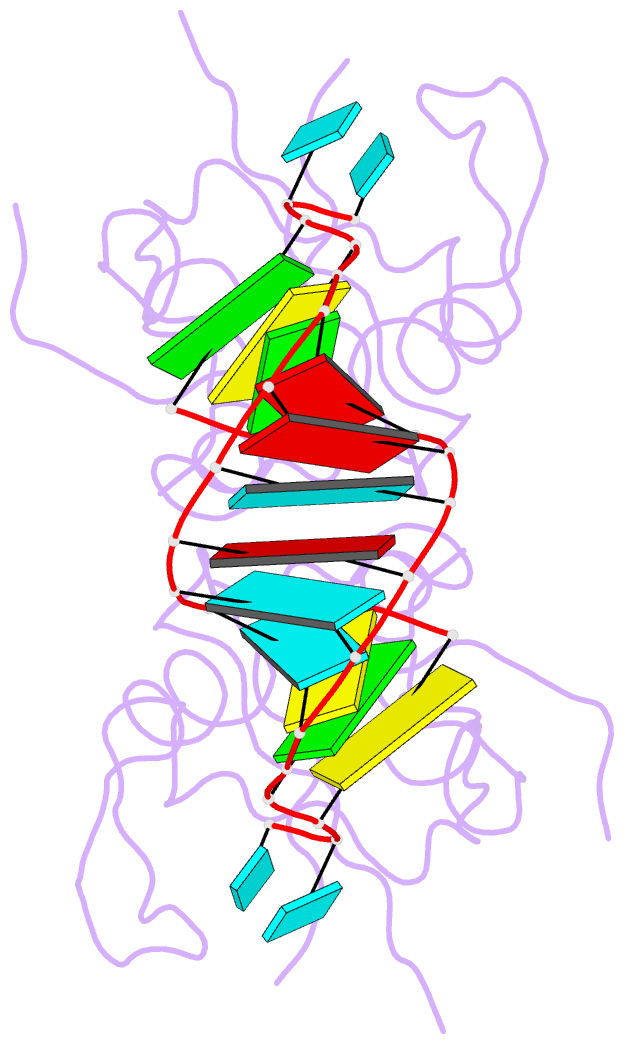
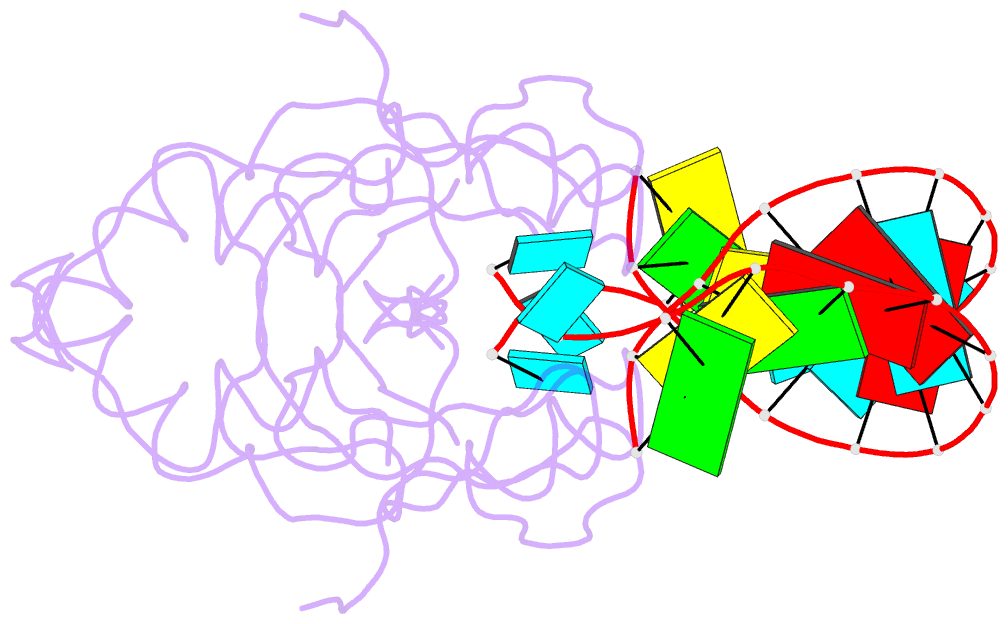
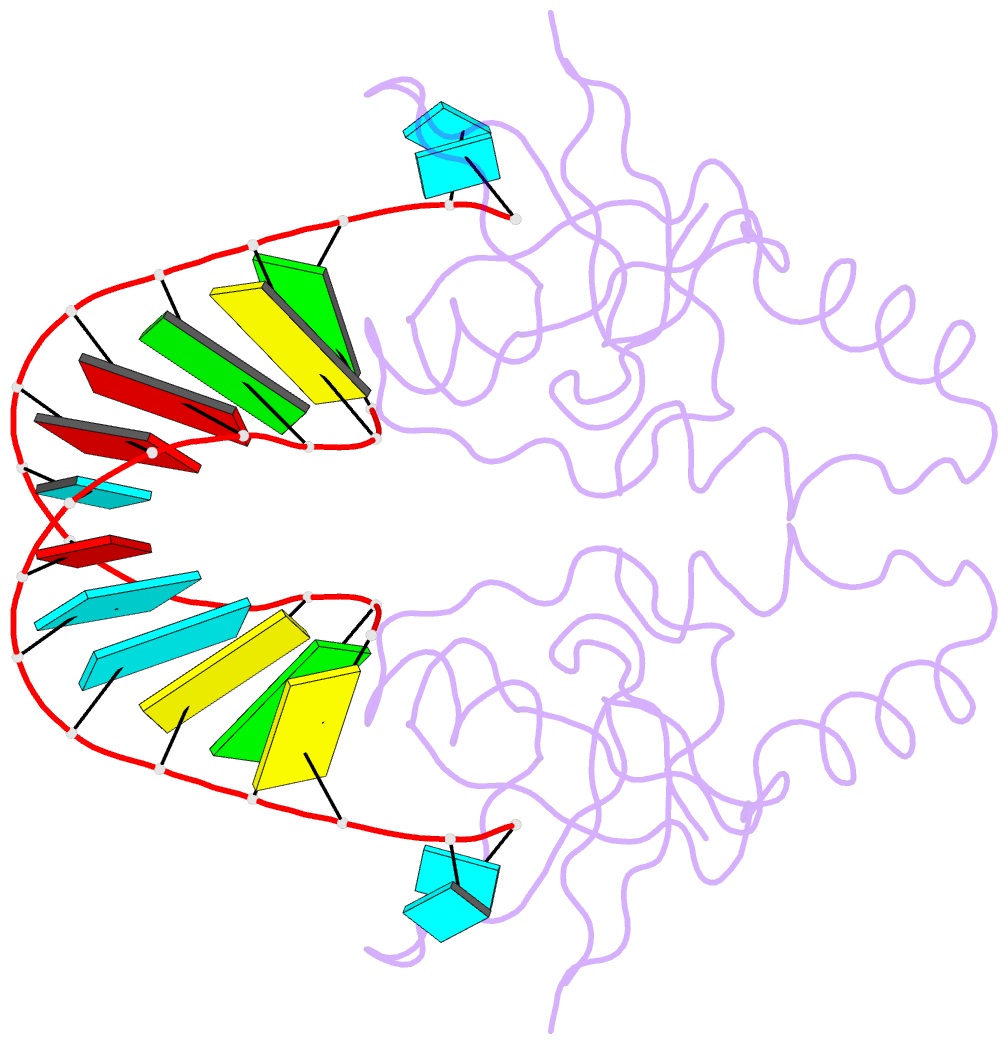
Summary information and primary citation
- PDB-id
- 3o6e; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (2.904 Å)
- Summary
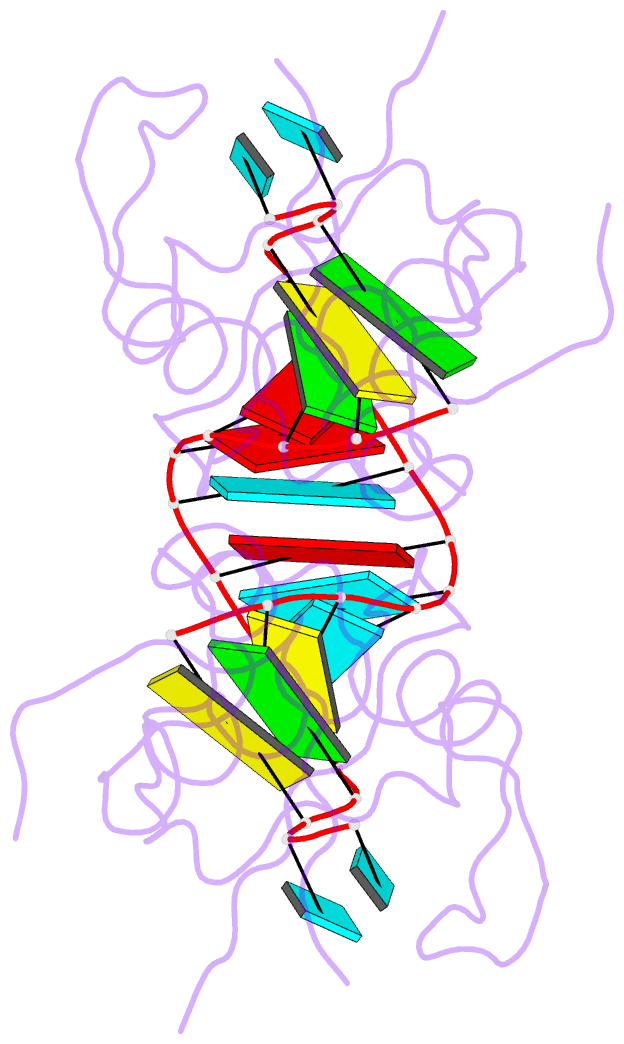
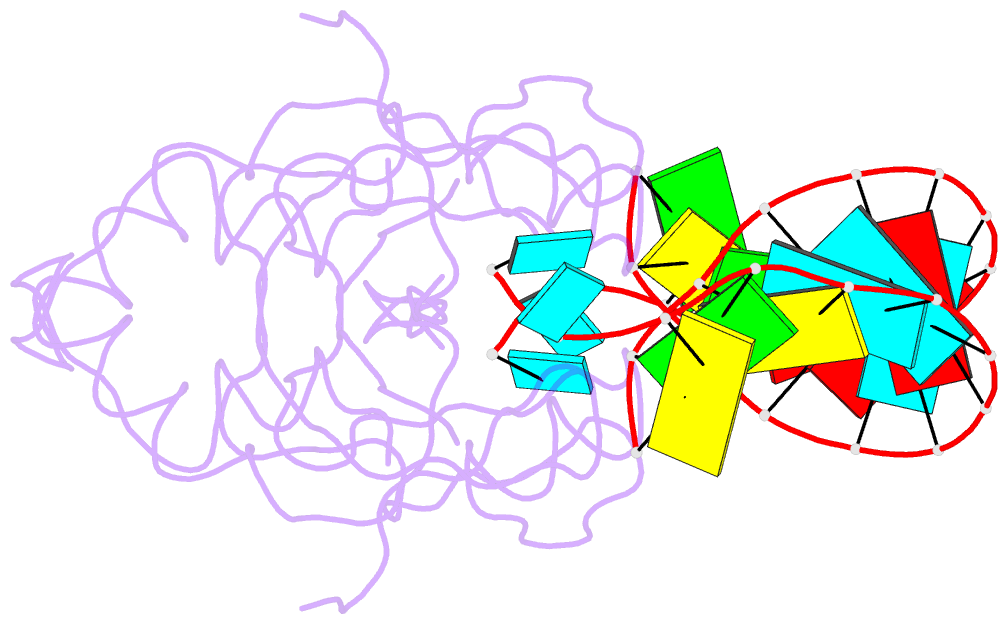
- Crystal structure of human hiwi1 paz domain (residues 277-399) in complex with 14-mer RNA (12-bp + 2-nt overhang) containing 2'-och3 at its 3'-end
- Reference
- Tian Y, Simanshu DK, Ma JB, Patel DJ (2011): "Inaugural Article: Structural basis for piRNA 2'-O-methylated 3'-end recognition by Piwi PAZ (Piwi/Argonaute/Zwille) domains." Proc.Natl.Acad.Sci.USA, 108, 903-910. doi: 10.1073/pnas.1017762108.
- Abstract
- Argonaute and Piwi proteins are key players in the RNA silencing pathway, with the former interacting with micro-RNAs (miRNAs) and siRNAs, whereas the latter targets piwi-interacting RNAs (piRNAs) that are 2'-O-methylated (2(')-OCH(3)) at their 3' ends. Germline-specific piRNAs and Piwi proteins play a critical role in genome defense against transposable elements, thereby protecting the genome against transposon-induced defects in gametogenesis and fertility. Humans contain four Piwi family proteins designated Hiwi1, Hiwi2, Hiwi3, and Hili. We report on the structures of Hili-PAZ (Piwi/Argonaute/Zwille) domain in the free state and Hiwi1 PAZ domain bound to self-complementary 14-mer RNAs (12-bp + 2-nt overhang) containing 2(')-OCH(3) and 2'-OH at their 3' ends. These structures explain the molecular basis underlying accommodation of the 2(')-OCH(3) group within a preformed Hiwi1 PAZ domain binding pocket, whose hydrophobic characteristics account for the preferential binding of 2(')-OCH(3) over 2'-OH 3' ends. These results contrast with the more restricted binding pocket for the human Ago1 PAZ domain, which exhibits a reverse order, with preferential binding of 2'-OH over 2(')-OCH(3) 3' ends.