Summary information and primary citation
- PDB-id
- 3oa6; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.35 Å)
- Summary
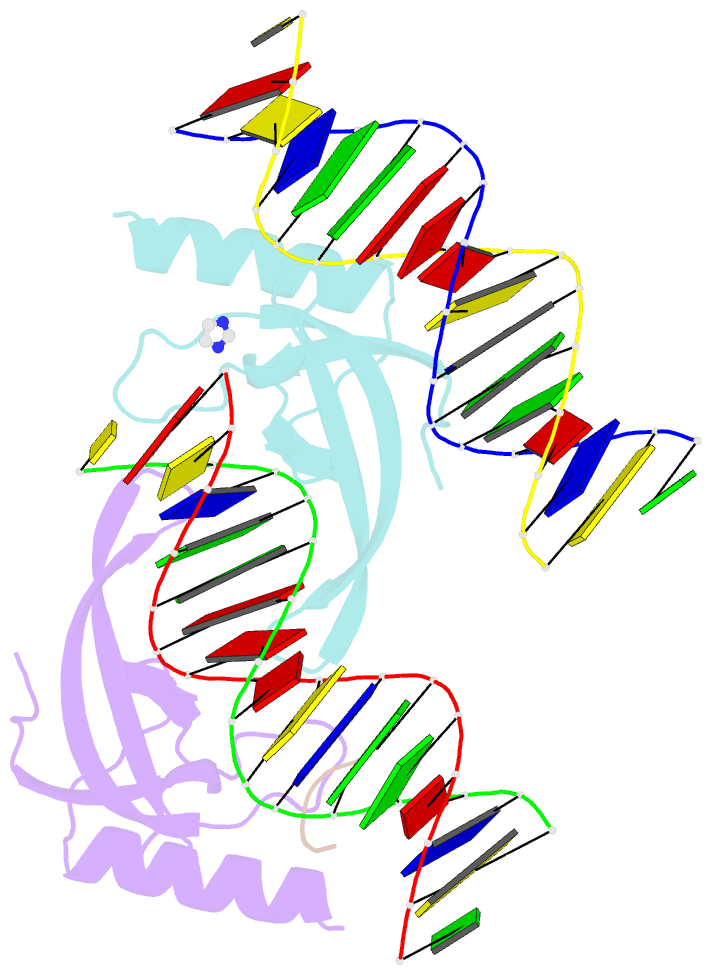
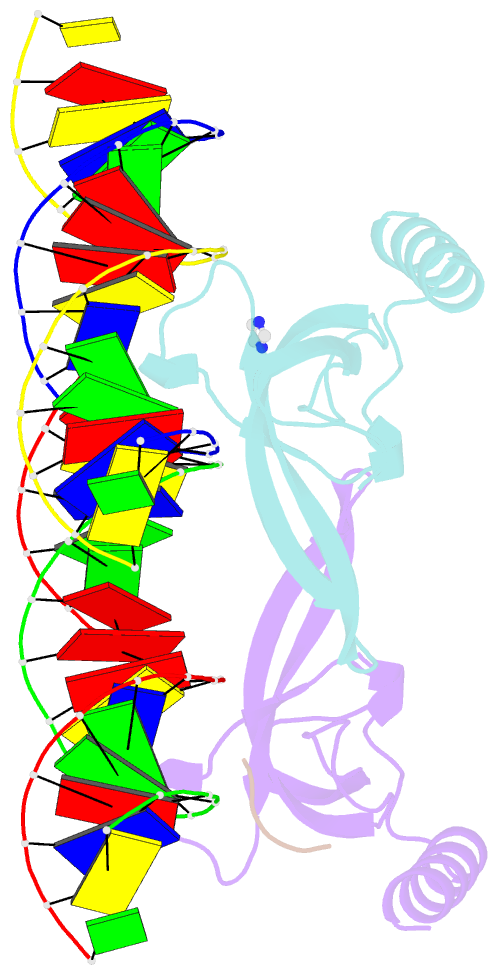
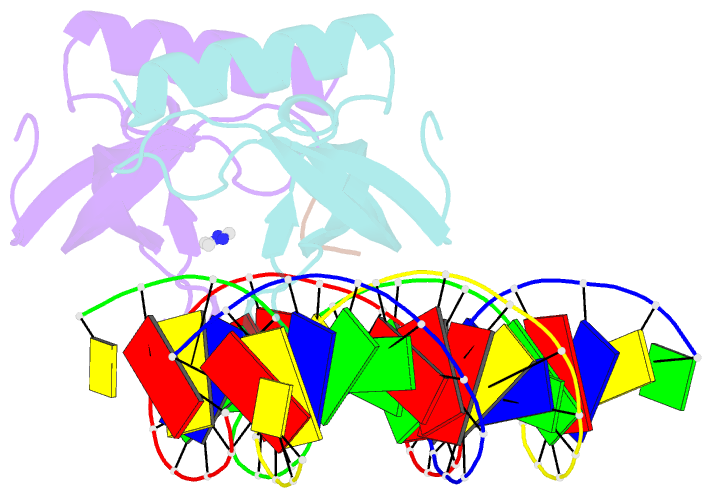
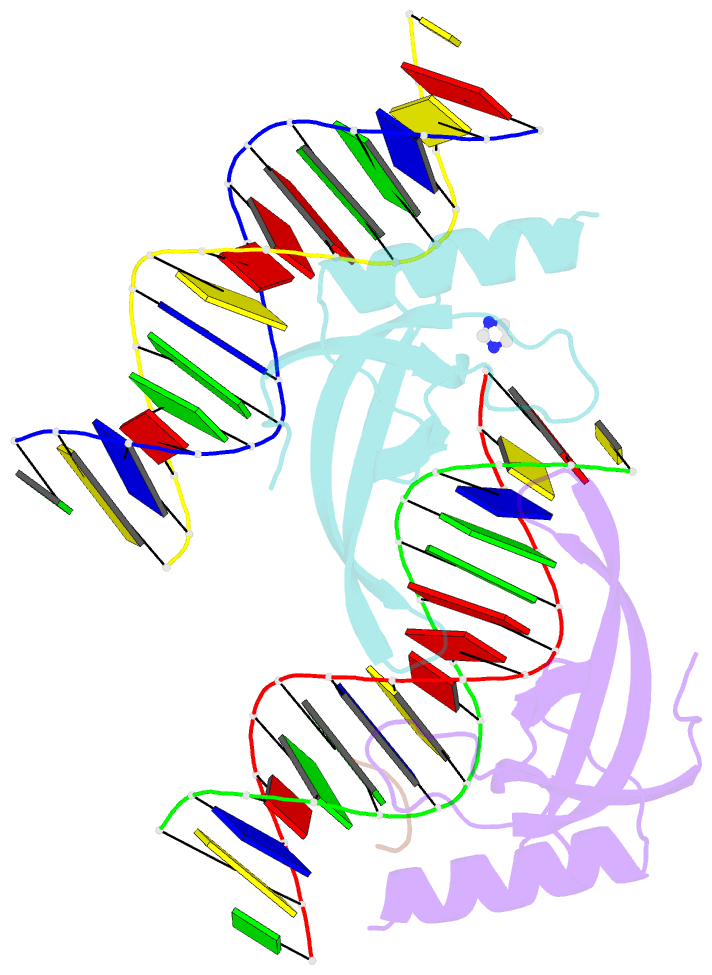
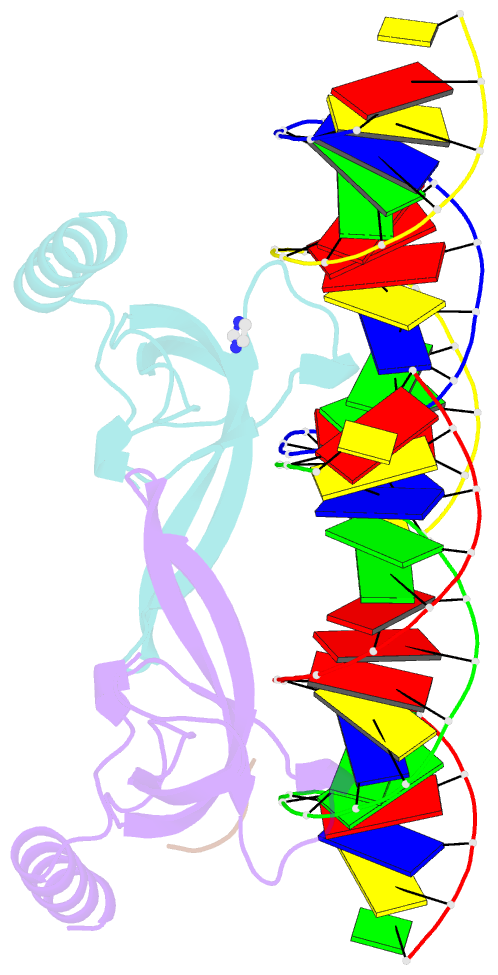
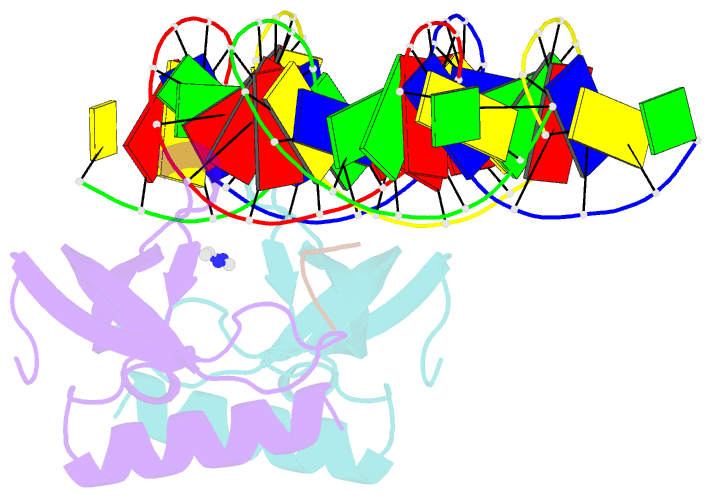
- Human msl3 chromodomain bound to DNA and h4k20me1 peptide
- Reference
- Kim D, Blus BJ, Chandra V, Huang P, Rastinejad F, Khorasanizadeh S (2010): "Corecognition of DNA and a methylated histone tail by the MSL3 chromodomain." Nat.Struct.Mol.Biol., 17, 1027-1029. doi: 10.1038/nsmb.1856.
- Abstract
- MSL3 resides in the MSL (male-specific lethal) complex, which upregulates transcription by spreading the histone H4 Lys16 (H4K16) acetyl mark. We discovered a DNA-dependent interaction of MSL3 chromodomain with the H4K20 monomethyl mark. The structure of a ternary complex shows that the DNA minor groove accommodates the histone H4 tail, and monomethyllysine inserts in a four-residue aromatic cage in MSL3. H4K16 acetylation antagonizes MSL3 binding, suggesting that MSL function is regulated by a combination of post-translational modifications.