Summary information and primary citation
- PDB-id
- 3on0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.874 Å)
- Summary
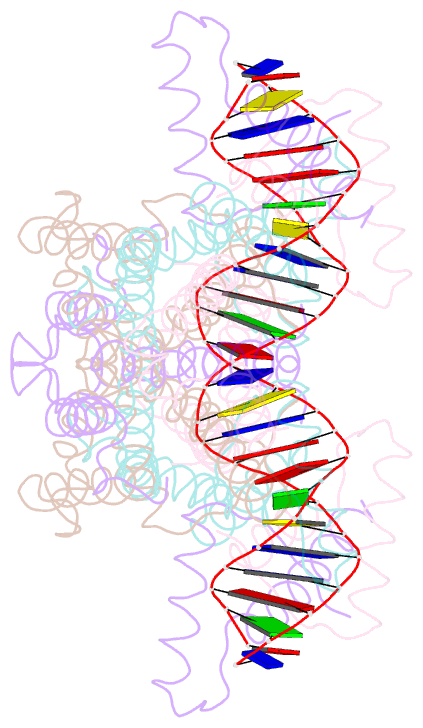
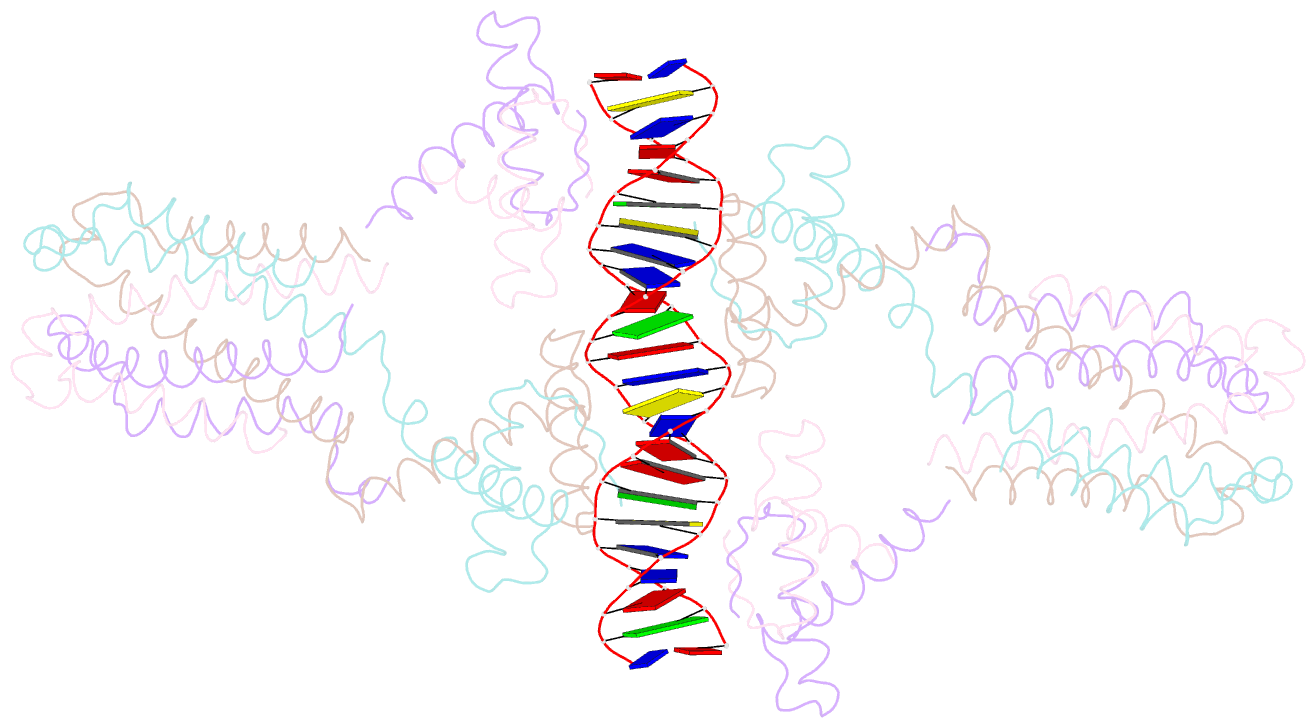
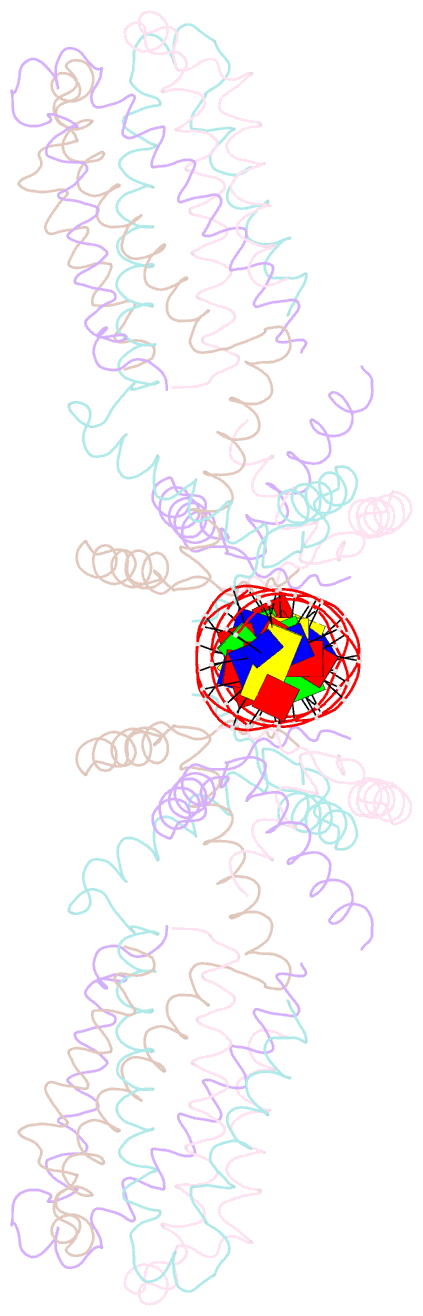
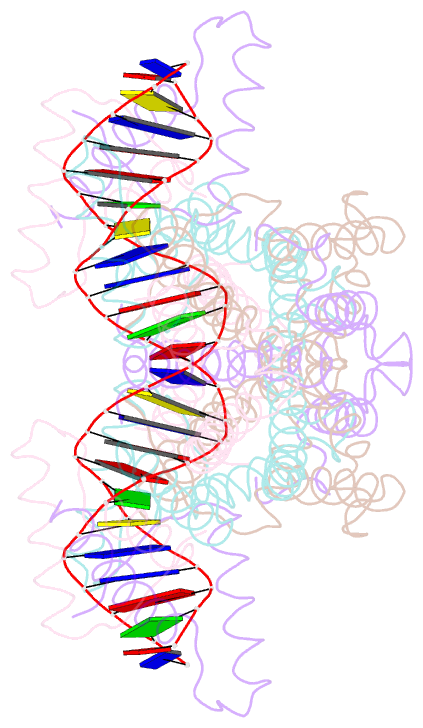
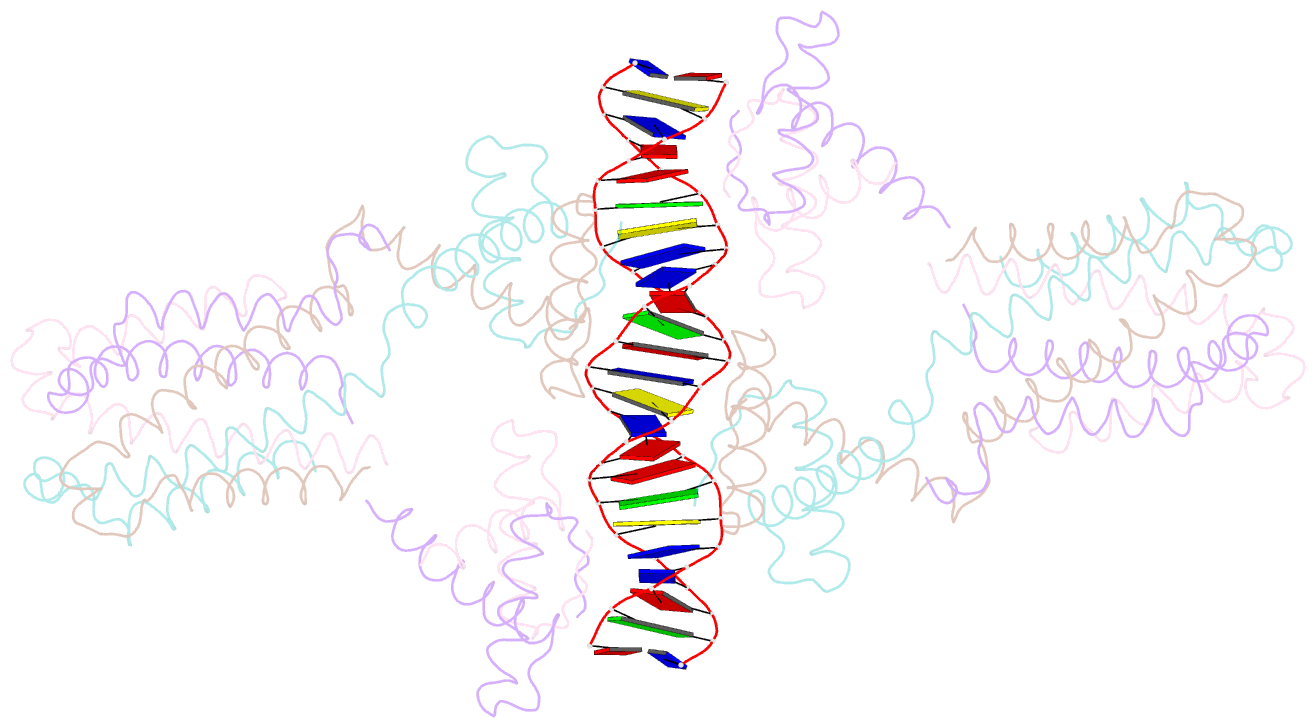
- Crystal structure of the ped208 tram-sbma complex
- Reference
- Wong JJ, Lu J, Edwards RA, Frost LS, Glover JN (2011): "Structural basis of cooperative DNA recognition by the plasmid conjugation factor, TraM." Nucleic Acids Res., 39, 6775-6788. doi: 10.1093/nar/gkr296.
- Abstract
- The conjugative transfer of F-like plasmids such as F, R1, R100 and pED208, between bacterial cells requires TraM, a plasmid-encoded DNA-binding protein. TraM tetramers bridge the origin of transfer (oriT) to a key component of the conjugative pore, the coupling protein TraD. Here we show that TraM recognizes a high-affinity DNA-binding site, sbmA, as a cooperative dimer of tetramers. The crystal structure of the TraM-sbmA complex from the plasmid pED208 shows that binding cooperativity is mediated by DNA kinking and unwinding, without any direct contact between tetramers. Sequence-specific DNA recognition is carried out by TraM's N-terminal ribbon-helix-helix (RHH) domains, which bind DNA in a staggered arrangement. We demonstrate that both DNA-binding specificity, as well as selective interactions between TraM and the C-terminal tail of its cognate TraD mediate conjugation specificity within the F-like family of plasmids. The ability of TraM to cooperatively bind DNA without interaction between tetramers leaves the C-terminal TraM tetramerization domains free to make multiple interactions with TraD, driving recruitment of the plasmid to the conjugative pore.