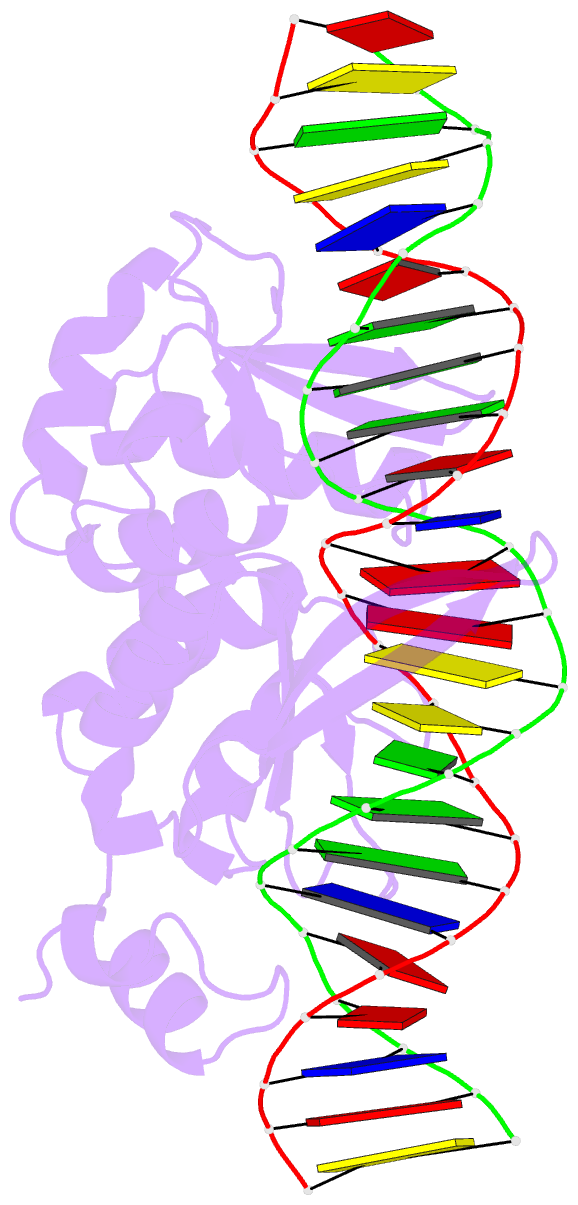
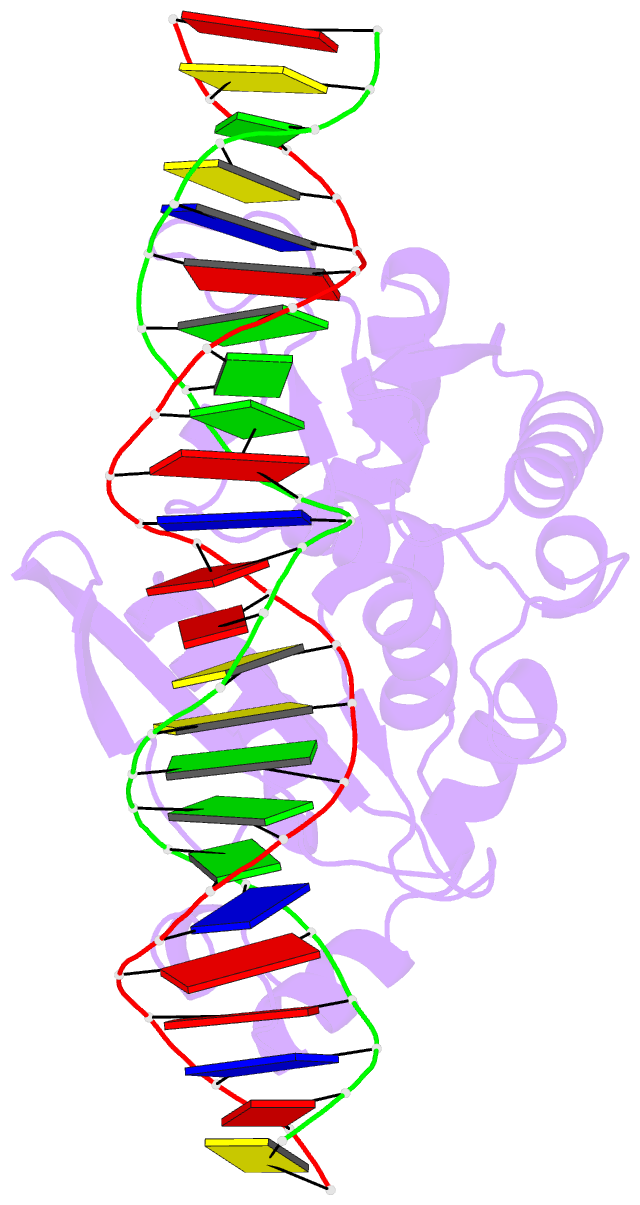
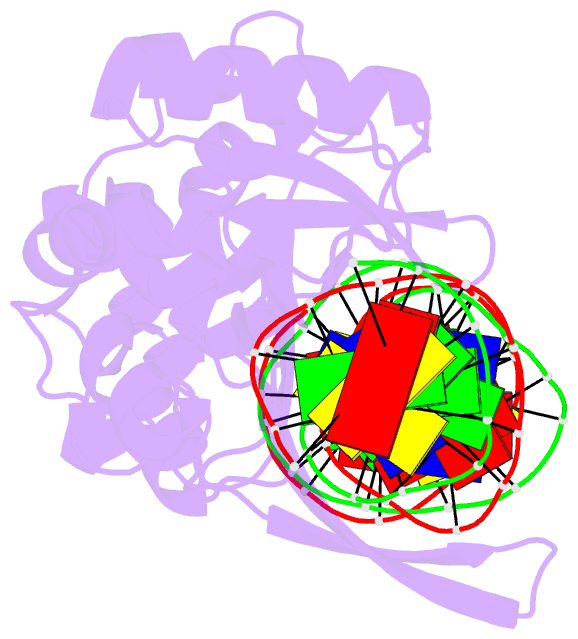
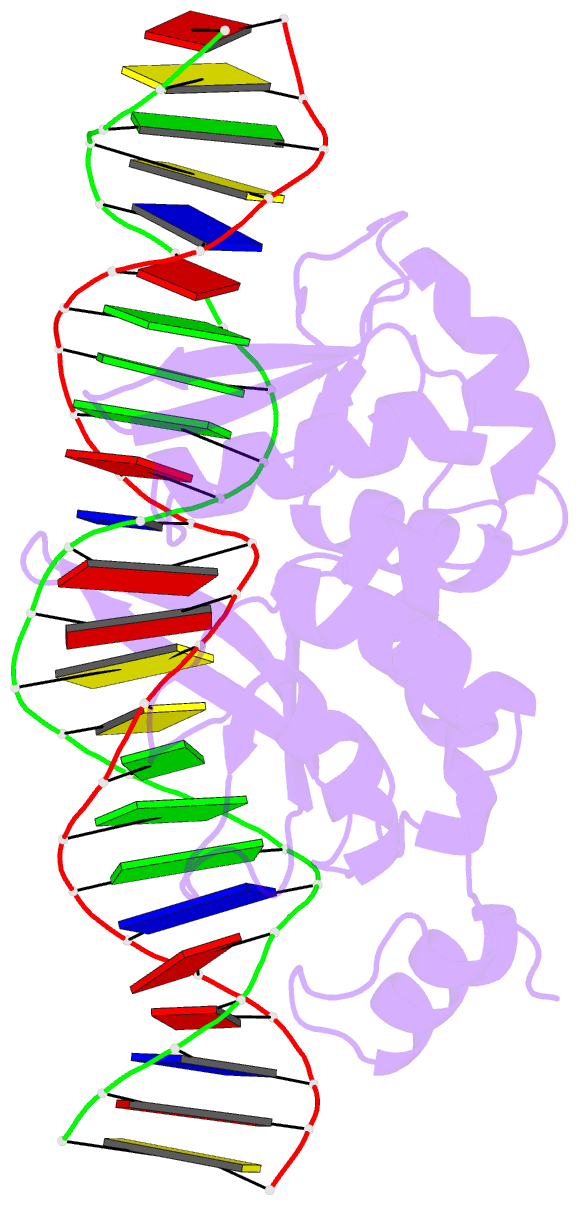
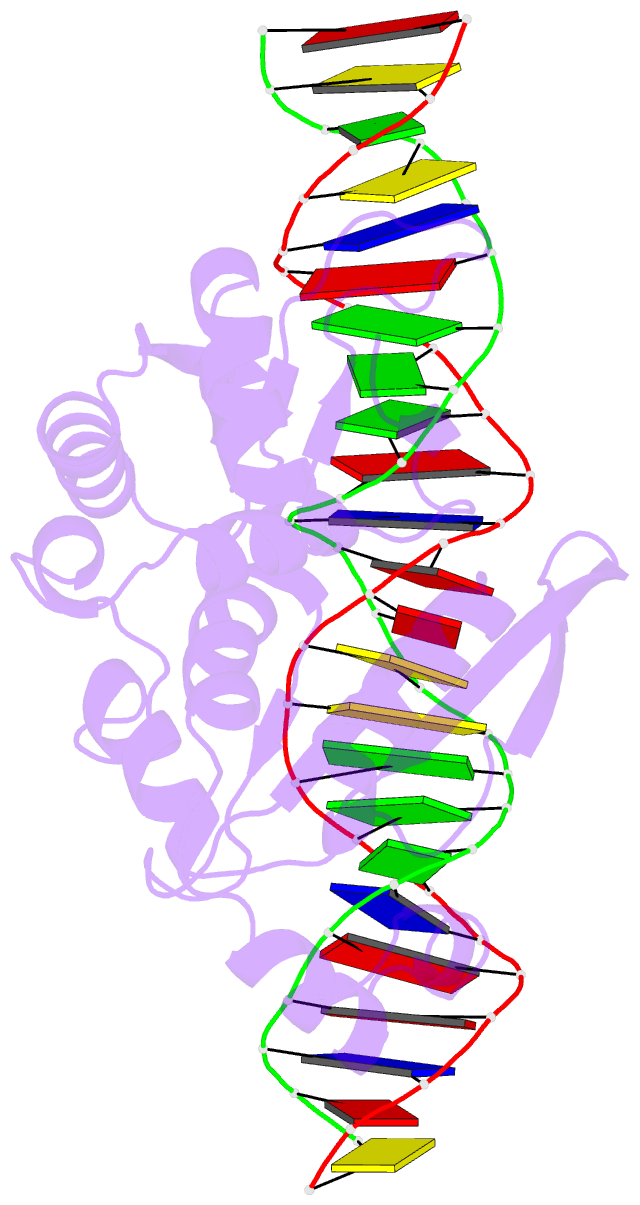
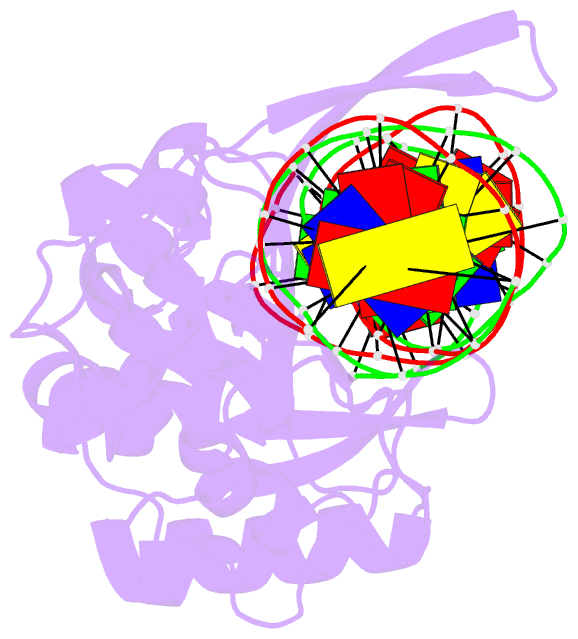
Summary information and primary citation
- PDB-id
- 3ool; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.3 Å)
- Summary
- I-scei complexed with c-g+4 DNA substrate
- Reference
- Joshi R, Ho KK, Tenney K, Chen JH, Golden BL, Gimble FS (2011): "Evolution of I-SceI Homing Endonucleases with Increased DNA Recognition Site Specificity." J.Mol.Biol., 405, 185-200. doi: 10.1016/j.jmb.2010.10.029.
- Abstract
- Elucidating how homing endonucleases undergo changes in recognition site specificity will facilitate efforts to engineer proteins for gene therapy applications. I-SceI is a monomeric homing endonuclease that recognizes and cleaves within an 18-bp target. It tolerates limited degeneracy in its target sequence, including substitution of a C:G(+4) base pair for the wild-type A:T(+4) base pair. Libraries encoding randomized amino acids at I-SceI residue positions that contact or are proximal to A:T(+4) were used in conjunction with a bacterial one-hybrid system to select I-SceI derivatives that bind to recognition sites containing either the A:T(+4) or the C:G(+4) base pairs. As expected, isolates encoding wild-type residues at the randomized positions were selected using either target sequence. All I-SceI proteins isolated using the C:G(+4) recognition site included small side-chain substitutions at G100 and either contained (K86R/G100T, K86R/G100S and K86R/G100C) or lacked (G100A, G100T) a K86R substitution. Interestingly, the binding affinities of the selected variants for the wild-type A:T(+4) target are 4- to 11-fold lower than that of wild-type I-SceI, whereas those for the C:G(+4) target are similar. The increased specificity of the mutant proteins is also evident in binding experiments in vivo. These differences in binding affinities account for the observed ∼36-fold difference in target preference between the K86R/G100T and wild-type proteins in DNA cleavage assays. An X-ray crystal structure of the K86R/G100T mutant protein bound to a DNA duplex containing the C:G(+4) substitution suggests how sequence specificity of a homing enzyme can increase. This biochemical and structural analysis defines one pathway by which site specificity is augmented for a homing endonuclease.